Research involving the use of nanomaterials for bone tissue engineering applications
Various bone fractures, osteoarthritis, osteoporosis or bone cancers represent common and significant clinical problems. Traditional orthopedic implant materials only last 10-15 years on average and implant failures originating from implant loosening, inflammation, infection, osteolysis and wear debris frequently occur. It is clearly urgent to develop a new generation of cytocompatible bone substitutes to regenerate bone tissue at defect sites that will last the life time of the patient.
The ideal orthopedic material would be one that exhibits the following properties: not evoke an inflammatory response disproportionate to its beneficial effect, degrade after fulfilling its purpose and leave no harmful components, be easily processed into the final product form, have an acceptable shelf life and be easily sterilized [1].
Nanoceramics
Nanoceramics such as alumina, zinc oxide (23nm) and titania (32nm) have shown increased bone growth over microphase alumina, zinc oxide (4.9µm) and titania (4.1µm) [2]. Metallic oxides (alumina, zirconia, titania), calcium phosphates (hydroxyapatite (HA), tricalcium phosphate (TCP), calcium tetraphosphate (Ca4P2O9) and glass ceramics (Bioglass, Ceravital) are among those commonly used in orthopedic tissue engineering applications. Their reaction with physiological fluids creates strong bonds to hard and soft tissues, thereby increasing osseointegration between implants and bone. Moreover, these ceramics are degradable and their dissolution rate depends on crystallinity. Therefore, the degradation of many ceramics can be controlled to match the rate of new bone growth [1].
In vivo studies on rats demonstrated that nanocrystalline HA accelerated new bone formation when coated on titanium and tantalum scaffolds 6 weeks after implantation when used as an osteoconductive coating compared to uncoated or conventional micron size HA coated tantalum [3].
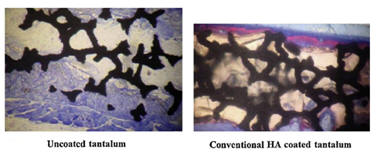
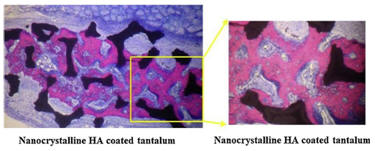
In vitro studies have shown that after just 4 hours in culture, 67nm HA significantly enhanced osteoblast adhesion and inhibited competitive fibroblast adhesion compared to conventional 179nm size HA. This is due to the high adsorption of vitronectin, a protein which promotes osteoblast adhesion, on the nanophase ceramic. Furthermore, enhanced osteoclast-like cell functions such as the synthesis of tartrate-resistant acid phosphatase (TRAP) and the formation of resorption pits have also been observed on nano-HA compared to micro-HA [4].
A biodegradable nanohydroxyapatite polyphosphazene microsphere 3-D scaffold was fabricated which had a compressive moduli of 46—81 MPa and cytocompatibility properties appropriate for bone tissue engineering applications [5].
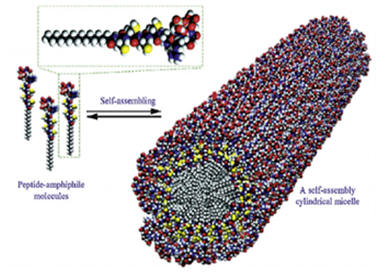
Supra molecular nanofibers consisting of HA aligned along the long axis of the nanofiber and consisting of a peptide-amphiphile (PA) with the cell-adhesive ligand RGD (Arg-Gly-Asp) was found to be similar to the bone pattern seen in vivo [6]. There was enhanced osteogenic differentiation of MSC in the 3-D PA scaffold in comparison to the 2-D static tissue culture. RGD modified PA nanofibers promoted the maximum amount of alkaline phosphatase activity and osteocalcin content by osteoblasts.
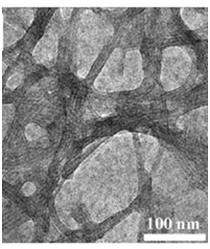
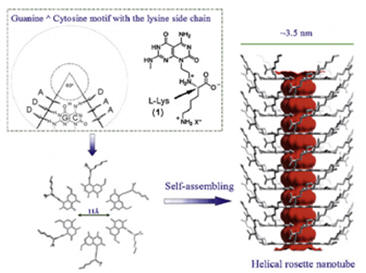
Osteogenic helical rosette nanotubes generated by the self-assembly of DNA base pairs Guanine and Cytosine in aqueous solutions have proved to be a promising bone substitute. They have modifiable amino acid and peptide side chains and allow for mineralization of biomimetic nanotube HA structures. Furthermore, significantly improved osteoblast adhesion has been observed on helical rosette nanotubes [7].
Carbon nanotubes/nanofibers (CNTs/CNFs) are good scaffold candidates for bone tissue engineering applications due to their superior cytocompatible, mechanical and electrical properties. It was seen that a 60nm diameter CNFs stimulated osseointegration by significantly increasing osteoblast adhesion and decreasing competitive cell adhesion [8]. CNT/CNF reinforced polymer nanocomposites have demonstrated excellent electrical conductivity for tissue regeneration. An 80%/20% polylactic acid (PLA)/CNT composite exhibited ideal electrical conductivity for bone growth in comparison to PLA which acted as an insulator [9]. Thus CNTs/CNFs can act as osteogenic scaffolds with superior properties to effectively enhance bone regeneration.


POLYMERS
Synthetic and natural polymers such as PGA, PLGA, PLLA, PLA, gelatin, collagen, chitosan etc. can be easily fabricated and are biodegradable, hence they are excellent candidates for bone tissue engineering applications. Furthermore, polymers can be modified chemically or functionalized via chemical and biochemical reactions.
Metals
Due to their high surface area, roughness, energy and load bearing ability, nanophase metals have been used for orthopedic applications. Nanophase Ti, Ti6Al4V and CoCrMo were found to significantly enhance osteoblast adhesion, morphology and alignment when compared to conventional metals [11]. Highly porous TiO2 nanotubes (diameter 100nm and length 500nm) layered on anodized Ti have shown to promote osseointegration in comparison to unanodized Ti in vitro [12].


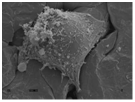


nanophase Ti nanophase CoCrMo nanophase Ti6Al4V unanodized Ti anodized Ti
"sMART" BIOACTIVE ORTHOPEDIC IMPLANTS
‘‘Smart’’ implants which, in addition to having good biocompatibility properties, have extended functionalities for drug delivery, in situ sensing, promoting tissue growth, etc. It allows for local and rapid drug delivery at the desired site. These implants facilitate smaller drug dosages, better control over toxicity and bioavailability of drugs, decreased antibiotic resistance, prolonged drug release and reduced systemic drug exposure [13].
References
[1] Nhiem Tran and Thomas J.
Webster.
Nanotechnology for bone materials. WIREs
Nanomedicine and Nanobiotechnology (2009), vol. 1, pp.
336-351.
[2] G. Colon, B.C. Ward, T.J. Webster. Increased
osteoblast and decreased Staphylococcus epidermidis
functions on nanophase ZnO and TiO2. Journal of
Biomedical Materials Research (2006), vol. 78, pp.
595-604.
[3] M. Sato. Nanophase hydroxyapatite coatings for
dental and orthopedic applications, PhD Thesis, Purdue
University, 2006.
[4] T.J. Webster, C. Ergun, R.H. Doremus, R.W. Siegel,
R. Bizios.
Specific proteins mediate enhanced osteoblast adhesion
on nanophase ceramics. Journal of Biomedical
Materials Research (2000), vol. 51, pp. 475 – 483.
[5] Syam P. Nukavarapu, Sangamesh G. Kumbar, Justin L.
Brown, Nicholas R. Krogman, Arlin L. Weikel, Mark D.
Hindenlang, Lakshmi S. Nair, Harry R. Allcock, Cato T.
Laurencin.
Polyphosphazene/Nano-Hydroxyapatite Composite
Microsphere Scaffolds for Bone Tissue Engineering.
Biomacromolecules (2008), Vol. 9, pp. 1818-1825.
[6]
Jeffrey D. Hartgerink, Elia Beniash, Samuel I. Stupp.
Self-Assembly and Mineralization of Peptide-Amphiphile
Nanofibers. Science (2001), vol. 294, pp. 1684-1688.
[7] Lijie Zhang, Felaniaina
Rakotondradany, Andrew J. Myles, Hicham Fenniri, Thomas
J. Webster. Biomaterials (2009), vol. 30, pp.
1309–1320. &. Lijie Zhang, Sharwatie Ramsaywack, Hicham
Fenniri, Thomas Webster. Enhanced Osteoblast Adhesion on
Self-Assembled Nanostructured Hydrogel Scaffolds.Tissue
Engineering: Part A (2008), vol. 14, pp. 1353-1364.
[8] Rachel L. Price, Michael C. Waid, Karen M.
Haberstroh, Thomas J. Webster. Selective bone cell
adhesion on formulations containing carbon nanofibers.
Biomaterials (2003), vol. 24, pp. 1877–1887.
[9] P.R. Supronowicz, P.M. Ajayan, K.R. Ullmann, B.P.
Arulanandam, D.W. Metzger, R. Bizios.
Novel
current-conducting composite substrates for exposing
osteoblasts to alternating current stimulation.
Journal of Biomedical Materials Research (2002), vol.
59, pp. 499-506.
[10] Jayarama Reddy Venugopal, Sharon Low, Aw Tar Choon,
A. Bharath Kumar, Seeram Ramakrishna. Nano bioengineered
Electrospun Composite Nanofibers and Osteoblasts for
Bone Regeneration. Artificial Organs (2008), vol. 32,
pp. 388-397.
[11] Thomas J. Webster, Jeremiah U. Ejiofor. Increased
osteoblast adhesion on nanophase metals: Ti, Ti6Al4V,
and CoCrMo. Biomaterials (2004), vol. 25, pp. 4731-
4739.
[12] Chang Yao, Venu Perla, Janice McKenzie, Elliot
Slamovich, Thomas Webster. Anodized Ti and Ti6Al4V
Possessing Nanometer Surface Features Enhances
Osteoblast Adhesion. Journal of Biomedical
Nanotechnology (2005), vol. 1, pp. 68-73.
[13] Phong A.
Tran, Love Sarin, Robert H. Hurt and Thomas J. Webster.
Opportunities for nanotechnology-enabled bioactive bone
implants. Journal of Materials Chemistry (2009), vol.
19, pp. 2653–2659.
