Similarities between bone tissue and nanomaterial bone substitutes
In addition to the dimensional similarity to bone, nanomaterials also exhibit unique surface properties such as surface topography, surface chemistry, surface wet-ability and surface energy, due to their significantly increased surface area and roughness compared to conventional or micron structured materials. These surface properties mediate specific protein (such as fibronectin, vitronectin and laminin) adsorption and bioactivity before cells adhere on implants, further regulating cell behavior and influencing tissue regeneration. Furthermore, an important criterion for designing orthopedic implant materials is the formation of sufficient osseointegration between synthetic materials and bone tissue. Studies have demonstrated that nanostructured materials with cell favorable surface properties may promote greater amounts of specific protein interactions to more efficiently stimulate new bone growth compared to conventional materials [1].
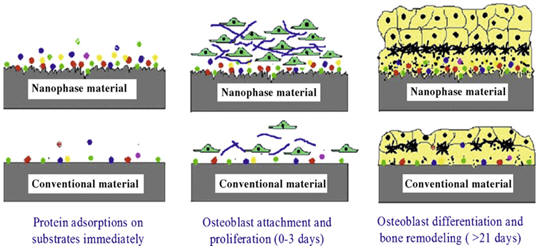
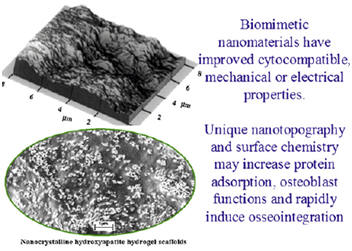
Nano phase ceramics, especially nano-hydroxyapatite, are popular bone substitutes, coatings and other filler materials due to their documented ability to promote mineralization. The nanometer grain sizes and high surface fraction of grain boundaries in nanoceramics increase osteoblast functions (such as adhesion, proliferation and differentiation).
Osteoproductivity induces integration between bone and an implant in the form of a continuous interfacial layer, while osteoconductivity only induces bone growth directly at the implant surface and often results in a fibrous capsule between the implant surface and bone. Porous scaffolds allow for better osteoconduction and bone integration by permitting new bone growth into the pores from the adjacent bone by incorporating cytokines or cells into these porous scaffolds they could be made osteoproductive by inducing osteogenesis. Osteoproductivity can be gained by incorporating a negative charge on an implant surface by metal oxide gel formation and also by manipulating the implant’s surface chemistry (such as placing carboxylate groups on the surface to chelate calcium ions). Such modification enhances the formation of apatite layers on increased negatively charged surfaces. With new capabilities to modify surfaces, osteoconductive implants are moving in the direction of osteoproductive implants by not only allowing the bone to grow at the surface but also integrating bone growth within the complex contour of an implant surface [2]
References
[1] Lijie Zhang, Thomas J. Webster. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today (2009), vol. , pp.66—80.
[2] Phong A. Tran, Love Sarin, Robert H. Hurt and Thomas J. Webster. Opportunities for nanotechnology-enabled bioactive bone implants. Journal of Materials Chemistry (2009), vol. 19, pp. 2653–2659.
