Case studies
Alveolar bone destruction, periodontopathic, jaw cysts etc. often leads to varying degrees of loosening of teeth which would need treatment ie. bone replacement. The bone is first augmented with replacement material and then the implant is made after approximately three months. Bone formation materials promote the formation of new bone and participate in osseous remodelling. They are degraded in parallel with bone regeneration and are completely used up after the defect has been reossified. Bone formation materials have osteoconductive properties allowing the migration of autologous osteoblasts, collagen fibres and capillaries into them [1].
NanoBone® (ARTOSS GmbH Rostock) is a synthetic bone replacement which utilizes cutting-edge nanotechnology to deliver a solution with similar strength and mechanical properties to bone. Its bio-active properties are due to the presence of hydroxyapatite crystals coated in silica molecules. By contacting the patients blood, body produced proteins will adhere to these nanopores [2].



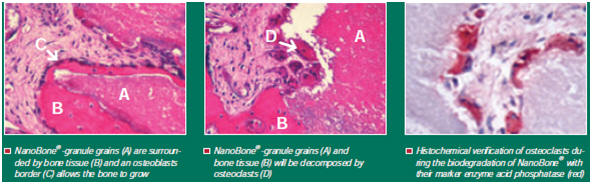
Its production is based on the sol-gel procedure. It is administered into an SiO2 solution in powdered form and is distributed homogeneously. In the gel transition phase a nanoporous matrix developing via SiO2 bonds connects the loosely packed hydroxyapatite crystals. The solvent escapes during the drying phase at a maximum temperature of 700°C allowing the formation of pores in the nm to µm size range. This results in a highly porous granulate with a porosity degree of approximately 60% similar to that seen in inorganic bone structure, with the result that excellent osteoconductive properties are achieved [1].
Case 1 [1]: A 54-year-old patient suffered from a residual cyst in his lower jaw in region 43–47.
The defect had to be filled with bone in order to accelerate the reossification process and prevent potential spontaneous fractures in the postoperative healing phase. Before the defect was filled, the biomaterial was thoroughly mixed with the patient’s blood.
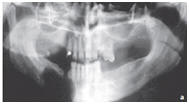
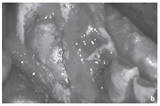
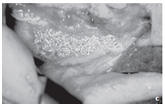
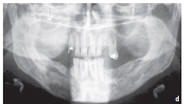
Figure: Large residual cyst in the lower jaw in region 43–47.
a. Preoperative orthopantomography representing the rarefaction of the bone
b. Intraoperative site prior to cyst removal
c. State after cystectomy and application of NanoBone® - shows the high capability of NanoBone® to remain fixed in place in the defect
d. Orthopantomography one month after the operation. Contrast- inducing residues of NanoBone® can be detected centrally in the defect region.
Case 2 [1]: A 37-year-old patient suffering from a profound parodontopathy in region 37.
Bone filling was proposed due to the threatened stability of the pier tooth 37. NanoBone® was applied into the periodontal defect after being mixed thoroughly with the patient’s blood.
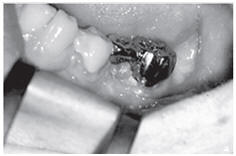
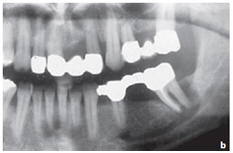
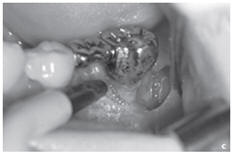
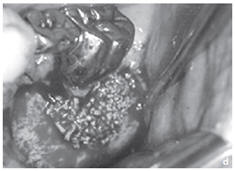
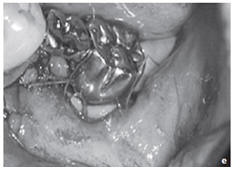
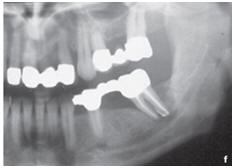
Figure: Profound parodontopathy with alveolar bone destruction in region 37.
a., b. Extent of bone destruction from preoperative clinical and radiological periodontal findings, region 37.
c, d. Surgical site prior and subsequent to the application of NanoBone®. Since the material is surrounded by bone in part only, its excellent capability to remain fixed in place is particularly evident in this case.
e.State after closing of the wound without any gaps. After the mucoperiosteal flap had been returned, the wound was closed without any gaps using a non-absorbable suture material.
f. Radiological results one month after the operation showed a minor bone apposition in addition to contrast-inducing residual biomaterial.
Conclusion
Bioceramics that are produced in the conventional manner are degraded slowly, often incompletely and can lead to resorptive-inflammatory reactions, which in turn impede the reossification process. Hence, they just comply with the characteristic features of a bone replacement material and are not a desirable bone replacement.
Synthetically produced NanoBone® when tested for its clinical usefulness in short-term and long-term animal experiments on the mandible of miniature pigs, showed a high osteoconductivity and biodegradation behaviour corresponding to the formation of new bone. Furthermore, it has a unique osteoprotective effect which supports bone regeneration with a lasting effect. Due to its high plasticity after being mixed with autologous blood, it can be easily handled during the operation and can remain fixed in place [1].
Neither postoperative inflammatory reactions nor loss of applied material has been observed in patients treated with NanoBone®. Hence, it can be classified as a good bone formation material for bone regeneration purposes.
BONE REGENERATION WITH PORPUS TITANIUM GRANULES AT PERI-IMPLANTITIS
References
[1] V. Bienengräber, Th. Gerber, K.-O. Henkel, T. Bayerlein, P. Proff, T. Gedrange. The clinical application of a new synthetic bone grafting material in oral and maxillofacial surgery. Folia Morphol. (2006), vol. 65, pp. 84–88.
[2] http://www.artoss.com/orthopaedie.html?L=1
