Contents
1. Introduction
2. Biolocical Diffusion
3. Diffusion Weighted Image Acquisition
4. Diffusion Tensor Image Measures
5. White Matter Tractography
6. References
1. Introduction
The broad spectrum of magnetic resonance (MR) contrast mechanisms makes MRI one of the most powerful and flexible imaging modalities for the diagnosis in the central nervous system (CNS). Especially contrast mechanisms addressing functionality (i.e., fMRI, DTI), are critical for pre- and intraoperative decisions that promote the safety of the procedure and assure a successful conclusion. Measurement of signal attenuation form water diffusion is one of the most important of these contrast mechanisms. Diffusion tensor imaging (DTI) may be used to map and characterize the three- dimensional diffusion of water as a function spatial location [1].
Furthermore,
many pathologic processes of the CNS influence the microstructual
composition
and architecture of effected tissues and lead to
changes in
diffusion properties. Since DTI is sensitive to chances at the cellular
and
microstructual level, methods of acquisition and analysis of DTI are
evolving
rapidly. In fact, the high dimensionality of the diffusion tensor
presents both
challenges and novel opportunities for describing, visualizing and
analyzing
diffusion measurements.
Diffusion is a random transport phenomenon, caused by Brownian motion, and describes the transfer of material (i.e., water) form on e spatial location to another dependent on time. The Einstein diffusion equation [3]
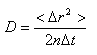 ,
(Eq. 1)
,
(Eq. 1)
states that the diffusion coefficient D (mm2/s) is proportional to the mean squared displacement <Δ r2> divided by the number of dimensions, n, and the diffusion time, Δt. At 20 °C the diffusion coefficient of pure water is 2.0 x 10-3 mm2/s and increases at higher temperature.
The
molecular water displacement can be described as a Gaussian probability
distribution:
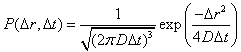 .
(Eq. 2)
.
(Eq. 2)
It
was
Basser at. al. [1], who
introduced the application of a diffusion tensor to
describe anisotropic diffusion behaviour. In this model, diffusion is
described
by a multivariate normal distribution:
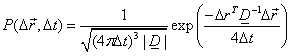 ,
(Eq. 3)
,
(Eq. 3)where
the
diffusion tensor is a 3 by 3 covariance matrix
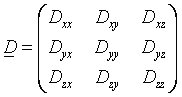 ,
,
The diffusion can be visualized as an ellipsoid (see Fig. 1), with the eigenvectors defining the direction of the principal axes and the eigenvalues defining the ellipsoidal radii. Diffusion is considered isotropic, if the eingenvalues are almost equal, and anistropic, if the eigenvalues are significantly different in magnitude.
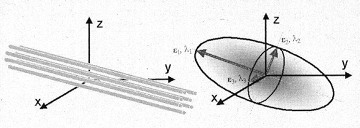
|
Fig. 1. Schematic
representation of diffusion displacement distributions for the
diffusion
tensor. Ellipsoids represent diffusion displacements. The diffusion is
highly
anisotropic in fibrous tissues such as white matter, and the direction
of
greatest diffusivity is generally assumed to be parallel to the local
direction
of white matter. |
The
eigenvalue magnitude may be affected by changes in local tissue
microstructure
including various types of injury, disease or normal physiological
changes
(i.e., aging).
3. Diffusion Weighted Image Acquisition
The simplest and most common approach to acquire diffusion weighted images using MRI is the pulsed-gradient spin echo pulse sequence with a single-shot, echo planar imaging readout, depicted in Fig. 2.
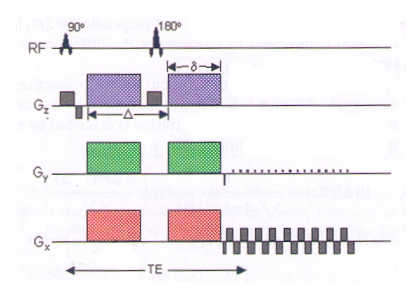
|
Fig. 2.
Schematic of a diffusion weighted echo-planar imaging (EPI) pulse
sequence. A
spin echo is used to achieve a diffusion weighted image from the
gradient pulse
pairs (colored
gradients on each side of
the 180° radiofrequency (RF) pulse). The normal EPI gradients are shown
in grey. |
The first gradient pulse (see Fig. 2, colored pulse on the left side of the 180° RF pulse) dephases the magnetization. the second pulse (see Fig. 2, colored pulse on the rightt of the 180° RF pulse) rephases the magnetization, and a spin echo is sampled afterwards. For non diffusing molecules, the phases induced by both gradient pulses will cancel out and there will be no signal attenuation. In contrast, if there is diffusion in the direction of the applied gradient, there will be a net phase difference, which is proportional to the displacement and dependent on the diffusion gradient pulse pairs of amplitude G, duration δ, and spacing Δ.
Due
to the
displacement of diffusing water, modelled by Eq. 2, water molecules at
each
voxel will accumulate different phases, and there will be signal
attenuation. For
the described pulse
sequence (see Fig. 2)
and isotropic Gaussian diffusion, the signal S can be described by:
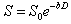 ,
(Eq. 4)
,
(Eq. 4)
where
S is the diffusion weighted signal, S0 is the signal
without any
diffusion weighted gradients, D is
the diffusion coefficient, and b is
the diffusion-weighting described by the properties of the pulse pair:
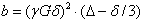
γ is the gyromagnetic ratio.
Even
though
EPI single shot is the most common acquisition method for DWI, there
are some major
problems including magnetic field inhomogeneities [4],
motion artefacts [5] and
eddy currents [6]. These
problems are often addressed by corrections in phase
direction, diffusion–weighting schemes, and image registration.
To measure the full diffusion tensor a minimum of six noncollinear diffusion encoding directions are required [7]. Using two- dimensional EPI pulse sequences at 1.5 T, a spatial resolution of 2.5 mm over the entire brain can be achieved in 15 minutes [8].
4. Diffusion Tensor Image Measures
The
interpretation,
measurement and display of a 3 by 3 diffusion matrix at each voxel is
an almost
impossible task
without simplification
of the data. Most commonly, the trace of a tensor (Tr),
the apparent diffusion coefficient MD,
or the fractional anisotropy FA is
used. Tr is simply
the sum of the eigenvalues of D, MD
is the average of eigenvalues of D,
and
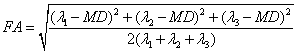 ,
(Eq. 6)
,
(Eq. 6)
which
was fist
described by Basser and Pierpaoli [9].
However, these measures may not be
applicable for every quantity of interest, described by the diffusion
tensor, and
may need be adapted accordingly.
A very common method in DTI is to display tensor orientation, described by the major eigenvector direction, as RGB color maps. For diffusion tensors with high anisotropy, the major eigenvector direction is generally assumed to be parallel to the direction of white matter tract, and the RGB color map is used to indicate the major eigenvector orientation.
Quantitative maps of MD, FA, and eigenvector measures are shown in Fig. 3 bellow:
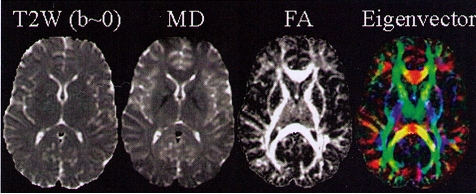
|
Fig 3. Quantitative
maps of DTI measurements. Left to right: T2-weighted (T2W) reference
image
(i.e., b=0), the mean diffusivity (MD; CSF appearing hyperintense),
fractional
anisotropy (FA; hyperintense in white matter), the major eigenvector
direction
indicated by RGB color map ( red: right-left; green:
anterior-posterior; blue:
superior inferior) [10]. |
Instead
of
simply using RGB color maps to visualize the orientation of the major
eigenvector (also see Fig. 3),
white matter tractography can be employed to display white matter
connection in 3D. White matter tractography algorithms follow coherent
spatial
patterns in the major eigenvectors of the diffusion tensor field.
Starting form
a specified location, the direction of propagation is estimated in
small subsequent
steps until the tract is terminated. Based on this method white matter
trajectories can be plausibly estimated and major projection pathways
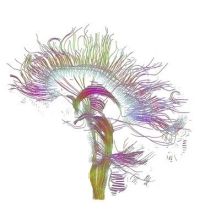
estimated (see Fig. 4).
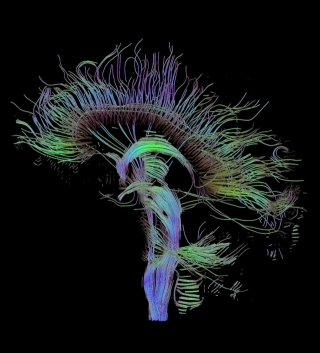
Fig.
4.
Reconstructed major fiber tracts in the mid sagi- tal plane
using white matter
tractography.