|
Single-Cell Isolation Array For Drug Screening and Drug Regimen Design |
| Principles of Single-Cell Analysis |
| Device Fabrication and Design |
| Uses in Clinical and Lab Settings |
| Further Reading |
| References |
| BME240 Home |
|
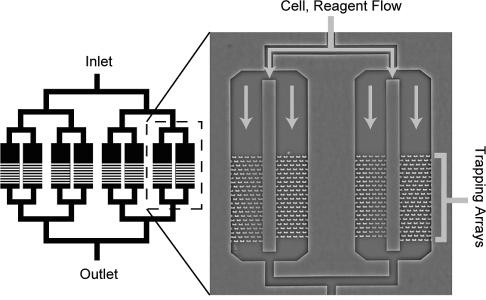
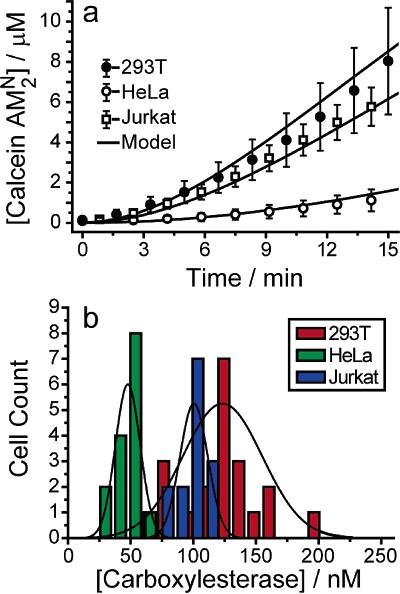
Device Applications It is important to note that while these devices are just applications of already-established ideas from the microfluidics community, they are still very novel and not ready for practical use by either scientists or physicians. The primary obstacle is that the devices are by no means user-friendly: the prototypes currently appearing in various chemistry or applied physics journals show that certain methods of applying chemicals to cells in an organized manner will work, but realistically, only if done by the professor who invented it. Ease of use will need to be greatly improved before acceptance, and even then, the target audience will be the research community first and the clinical one second. Nonetheless, it is possible to speculate on possible clinical applications for the years to come, as the microfluidics approach is currently the most promising plan towards cheap, fast, and accurate assays using a minimal amount of reagent. The current trend is to work towards the incorporation of microfluidic chips into first, the diagnosis of disease, and second, the design of patient-specific drug regimens based on the results of the diagnosis. Below is a general proof-of-concept for the use of microfluidic single-cell arrays in drug analysis. Carboxylesterase Kinetics for Drug Uptake Various types of cancer cells were stained with calcein AM, which fluoresces in response to contact with intracellular enzymes such as carboxylesterase, and then loaded into an array. NDGA is applied to the cells to inhibit carboxylesterase, and under a microscope, a CCD is used to collect fluorescence data over time from the trapped cells. Corresponding kinetics models are then generated, as well as distributions of enzyme concentrations in various cell types. Because carboxylesterase is involved in activation and deactivation of various drugs, this simple experiment, designed to validate a device’s function more than anything else, provides a very relevant example of the device’s use in clinical medicine.
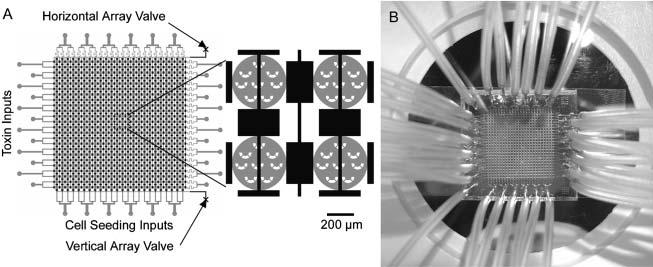
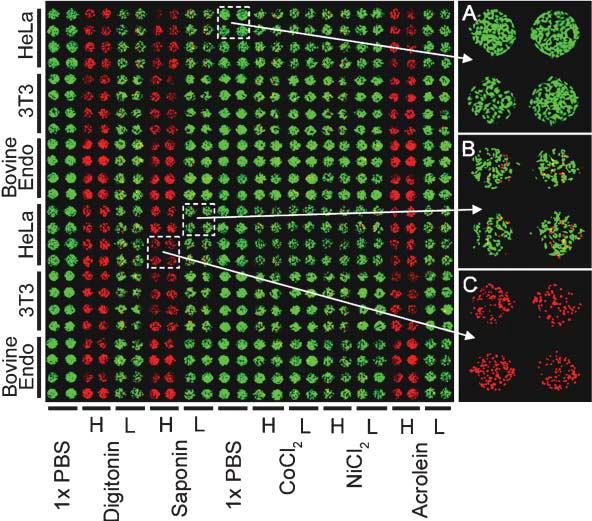
Cytotoxicity Assays Using Hepatocytes A specialized application of the above assay is the cytotoxicity analysis, a common goal for microfluidics-based cell assays. This application was designed with the pharmaceutical industry in mind, though it very easily translates to a practical device for a pharmacist to deduce a personalized cocktail for a patient requiring aggressive treatment, such as chemotherapy. To perform the assay, the device is seeded with hepatocytes, obtained perhaps via a liver biopsy. Depending on the layout of the channels, various drug concentrations can be generated and multiple drugs can be mixed to form a large combination of possibilities. We can then perform a simple live-dead analysis on the cells, or go further and check for cytochrome p450 activity, the active toxin metabolizer in hepatocytes.
|