The technological advances made within spinal surgery have increased dramatically within the past two decades. Although innovations in minimally invasive spine surgery continue to advance, these procedures have yet to gain widespread adoption due to the challenges of mastering the endoscopic technique. Difficulties remain in achieving dexterity and precision of instrument control within the confines of a limited operating space, further compounded by the need to operate from a 2D video image. The application of robotic technology has the potential to contribute significantly to the advancement of endoscopic spine surgery.
Every year, over 500,000 spine surgeries are performed in the US alone, with an average growth rate of 8% per annum. This necessitates the development of large technology base in mechanical design, kinematics, control algorithms, and programming so as to build efficient and precise robotic assist systems with enhanced capabilities and adaptabilities.
Application of Robotics to Endoscopic Spine Surgery
There are several distinct and compelling advantages associated with the use of surgical robot, which suggests that this particular technology is capable of significantly enhancing current operative technique. Unlike conventional instrumentation which requires manipulation in reverse, the proportional movement of the robotic device allows the instruments to follow the movement of the surgeon’s hands directly. The intuitive control of the instruments is particularly advantageous for the novice endoscopist. In addition to mimicking the surgeon’s movements in an intuitive manner, the robotic instruments offer six degrees of freedom plus grip, two more than conventional instruments. This technology permits a large range of motion and rotation that follows the natural range of articulation of the human wrist and may be particularly helpful when working space is limited. The electronic control system is capable of filtering out hand tremors as well as motion scaling, whereby gross hand movements at the surgeon’s console may be translated to much finer movement of the instrument tips at the operative site. The 3D vision system adds a measure of safety and surgical control beyond what is available with the traditional endoscope. The 3D display improves depth perception, and the ability to magnify images by a factor of 10 allows extremely sensitive and accurate surgical manipulation. The alignment of the visual axis with the surgeon’s hands in the console further enhances hand-eye coordination to a degree uncommon in traditional endoscopic surgery.
Spinal Surgical Procedure without the Help of Robotic Systems
As shown in the figure below, fusion is a surgical technique in which one or more of the vertebrae of the spine are fixed by connecting fixtures and rods together to restrict relative motions between them.
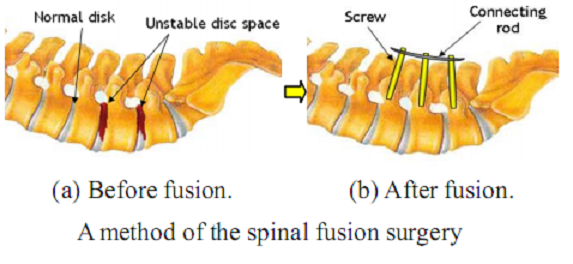
The spinal surgical procedure performed by surgeons without any help of robotic systems is as follows. In the preoperative stage, CT or MRI images around the surgical area of the patient are scanned and analyzed. Then, a proper surgical plan is established on the images. The area of the surgical operation is sterilized and dissected first in the intra-operative procedure. The surgeon bores guide holes in the vertebrae and inserts screws through them into the vertebrae. In these processes, the surgeon has to monitor fluoroscopic images to make sure that the instruments are on the pre-planned or the desired position. Once all screws required are inserted into the targeted pedicles, those screws are fixed together firmly with a connecting rod. Lastly, the wound is closed and sutured.
Problems
Most of the difficult tasks of the spinal fusion are on the process related to inserting screws. Generally, the average diameter of lumbar pedicles is slightly bigger than 6mm and the other pedicles are a little smaller than that. Pedicle screws sized to occupy 70% of pedicle diameter are normally used in the spinal fusion. Therefore, the surgeon should carefully insert a screw while paying his maximum attention to the fluoroscopic images available in the surgical operation because there is a small margin of error. Even a slight deviation from the center of the pedicle might cause breakage of a portion of edges of the pedicle into pieces. And the spinal nerve might be damaged, which causes catastrophic results to patient. Also, in the conventional fusion operation, the use of intra-operative fluoroscopy for the placement of pedicle screws has resulted in prolonged fluoro time and radiation exposure to the surgeon and the patient. The X-ray exposure time to the patient as well as to the surgeon is 0.33 minutes per screw, which is enormous. In addition, the percentage of misplaced pedicle screws that have more than 2mm deviation, is reported 3~55 % in the conventional method. The main reason for incorrect placement of a pedicle screw is probably because of the invisible body of the pedicle being penetrated in the intra-operative operation.
Some tools and robotic systems have been developed to reduce these unintentional deviations by guiding the instruments and to improve accuracy by targeting the desired position. The systems can be instrumental in improving accuracy and reducing the X-ray exposure time and unintentional deviation error.
Miniature Robotic Guidance for Spine Surgery
Instrumented spinal fusion surgery is increasingly performed in the treatment of mechanical back pain due to degenerative disc disease, spondylolytic spondylolisthesis, trauma and tumors affecting the spine. Breaching of the pedicle occurs in 3–55% of screws; clinically significant screw misplacements occur in 0–7% of all transpedicular screw placements. Neuromonitoring, neuro-stimulation and computer-assisted navigation systems reduce the incidence of screw misplacement; however, none of them has gained significant popularity in spine surgery, mainly due to logistical and cost-effectiveness issues, such as the need for dynamic referencing and a line-of-sight, extra staff, expensive tools and cumbersome procedures, longer operation time and the high cost of the capital equipment. Surgical robots emerged during the 1990s and offer distinct added value in terms of accuracy and minimal invasiveness of the surgical procedure. However, current systems are extremely expensive and large in size, and typically require immobilization of the patient.
An Image-Guided Robotic System for Spinal Fusion
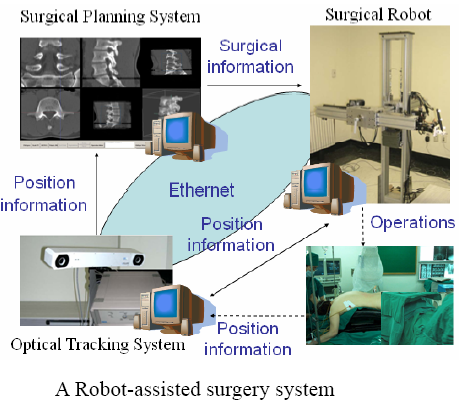
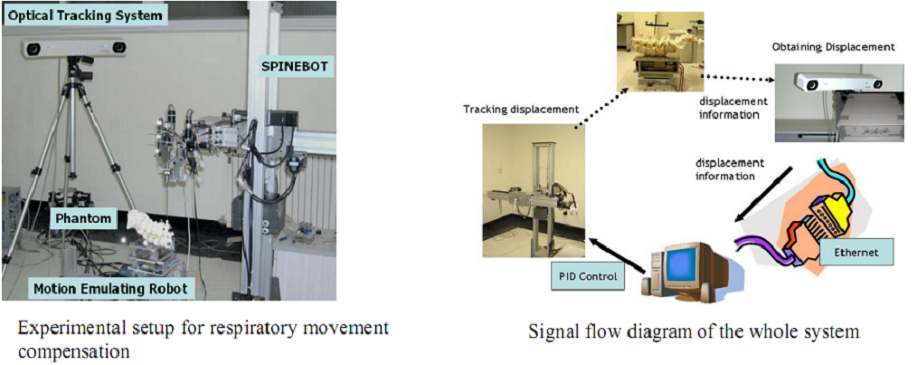
As shown in figure above, the constructed robot-assisted surgery system consists of a surgical planning system (HexaView), a surgical robot (SPINEBOT), and an optical tracking system (see figures below). In the preoperative and intra-operative procedures, the optical tracking system and the surgical planning system play the role of a navigation system. All sub-systems share and transfer data through Ethernet.
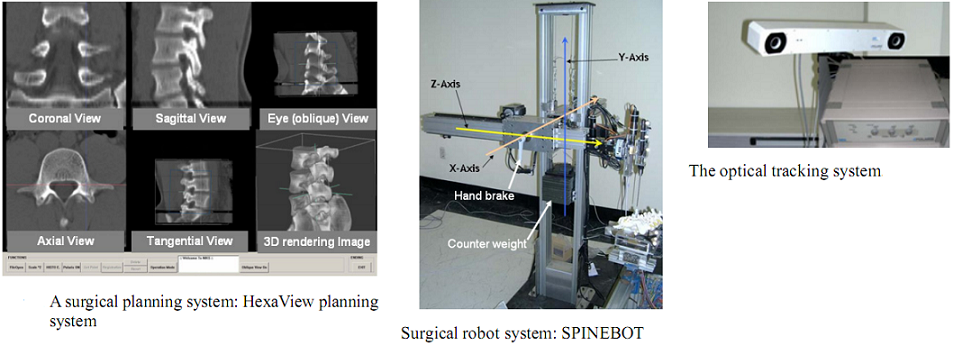
To align the coordinates of the system components, the optical tracking system is used to obtain the positions of the components in the real world. Using the pre-obtained images of the surgical area, the surgeon determines a desired operational path of the screw on the surgical planning system. The optical tracking system detects the movement of the surgical area by tracking the position of a probe attached to the surgical area. Both information of the operational path and the movement of the surgical area are transferred to the robot and then the robot conducts the operation while compensating the movement of the surgical area.
In spinal fusion, the system plays the role of assisting surgeon at several stages. The first and simplest role of the system is to guide surgical tools to perform the screw insertion operation by the surgeon easily. The second role is to perform a task of boring a guide hole in the lumbar. The third role is to conduct a task of inserting screws into the vertebra automatically (see figure below).
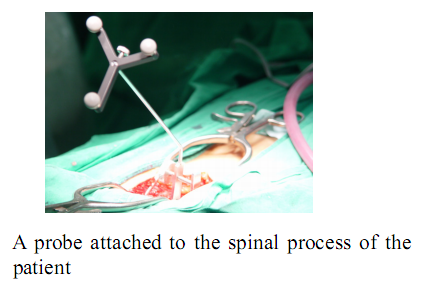
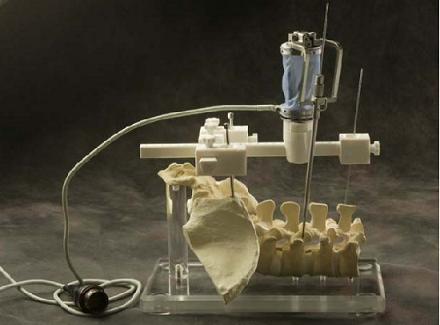
During actual spinal operation, the surgical area is continuously moving due to both the patient’s respiration and external screwing forces by the surgeon. The OTS detects the movement of the spinal process by tracking the probe. The movement of the patient’s lumbar due to his/her respiration is not negligible and needs to be compensated by some means, in order to perform more accurate spine surgical operation successfully. Three different approaches could be considered as compensation methods for the patient’s movements in surgical operation and the movements may not be necessarily due to the respiration of the patient. The first method is that the robot compensates the movement by directly following the respiratory movement of the human body, the second one is that the surgical bone is fixed firmly to some type of fixtures, and the third one is that the surgical robot is attached to the surgical bones. Figure below (left) shows the mockup setup for respiratory movement compensation (first method). In the mockup experiment, the respiratory motion emulating a human respiratory movement is generated by using a robot named, Motion Emulating Robot (MER). To locate the exact surgical position, the OTS measures the movement of MER continuously by tracking a probe attached on MER. The measured data is feedback to SPINEBOT and SPINEBOT is commanded to track the target position on MER. Another probe is attached to SPINEBOT to obtain the transformation between the OTS coordinates and the robot coordinates. The figure below (right) shows the signal flow diagram of the whole system employed in mockup experiment. The signal output from the OTS is directly sent to the SPINEBOT controller via Ethernet and low-pass filtered.
Procedure using SpineAssist
“SpineAssist” is a novel spine surgery miniature robot, developed by Mazor Surgical Technologies Ltd. Israel, that has been FDA approved and is extensively used in a wide variety of spine procedures. It facilitates image-based semi-active guidance for providing high accuracy in the insertion of implants, e.g. pedicle screws.
The SpineAssist (SA) is a bone-mounted robot (2.5 in diameter; 250 g) featuring a six degrees of freedom parallel design. The miniature robot is connected to the SA workstation (see figure below), which controls its motion and runs specially designed graphic user interface software. The system is semi-active, in that it guides the surgeon to the desired implant positions according to his/her preoperative plan, while leaving the actual surgical act in the physician’s hands. The concept is of preoperative planning and intraoperative execution. The planning is done on a three-dimensional (3D) model of the patient’s spine generated by the system, based on a CT scan. The plan includes implant placements for all the levels of the spine to be operated on, and can be done on the workstation itself or on the physician’s laptop or desktop computer.
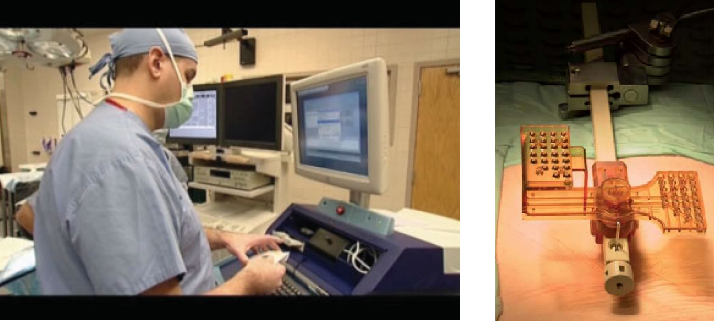
In preparation for the intraoperative execution of the plan, the SA workstation is connected by means of a BNC video cable to a C-arm fluoroscopy imaging machine and two blank images, anterior–posterior (AP) and lateral (LAT), are taken using a special image calibrator attached to the image intensifier of the C-arm. These two ‘blank’ images are used by the system to automatically compensate for distortions due to ambient magnetic fields and other sources of distortion to the intraoperative fluoroscopy images. The miniature robotic device is also verified for calibration prior to every case, using a specially designed jig with three marker holes at positions that are known to the software. The entire process of image and robot calibration takes about 10 min and is performed by the radiology technician during the set-up of the operating room for surgery, in parallel to other preparations and prior to bringing in the patient.
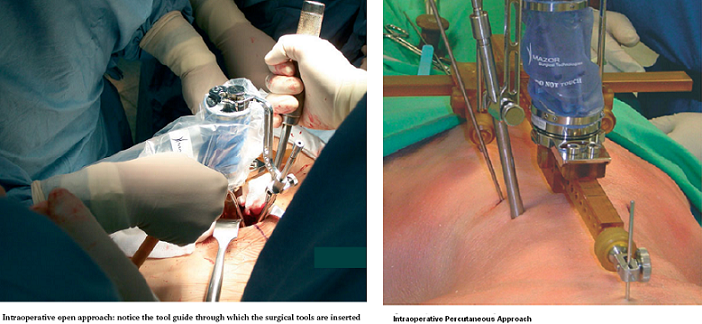
As the operation begins, a minimally invasive Hover-T frame or a less invasive spinous process clamp are attached to the patient’s bony anatomy. Four fluoroscopic images are taken, 2 AP and 2 LAT, with and without targeting devices attached to the Hover-T/clamp. The system performs automatic, per vertebra merging of these intraoperative fluoroscopic images with the preoperative CT. The accuracy of the image registration process is visually verified by the surgeon and the first level to be operated on is chosen. The SpineAssist device is mounted onto the clamp/frame and the system controls its motion so that it points to the exact entry point and trajectory, according to the surgeon’s preoperative plan. Based on the known kinematic properties of the system and the desired entry point relative to the robot base, the system instructs the surgeon to attach one of three guiding arms (short, medium or long) to the top plate of the robotic platform, through which surgical tools are inserted by the surgeon to facilitate introduction of the implant. The three arms cover the entire workspace necessary for a variety of spinal procedures. An open or percutaneous approach may be used (see figures below).
Robot-Assisted Verteboplasty
The prevalence of vertebral compression fractures (VCF) steadily increases with age, reaching 40 percent in women 80 years of age. VCF is the result of failure of the anterior column by forward flexion forces and is rarely associated with neurological deficits. Osteoporotic VCFs tend to cluster around T8 -T12 and L1-L4. Complications from compression fractures include: prolonged inactivity and pain, progressive muscle weakness, kyphosis and loss of height, crowding of internal organs, bowel obstruction, increased nursing home admissions, and mortality. Vertebroplasty involves percutaneous injection of acrylic cement or PMMA - polymethylmethacrylate into the vertebral body, usually through a transpedicular route. The steps are: (i) Fluoroscopic guided pin insertion through the pedicle into the anterior half of the vertebral body; (ii) Injecting the PMMA cement using continual fluoroscopic monitoring to prevent overfilling or extension into the spinal canal or neural foramen (see figure below).
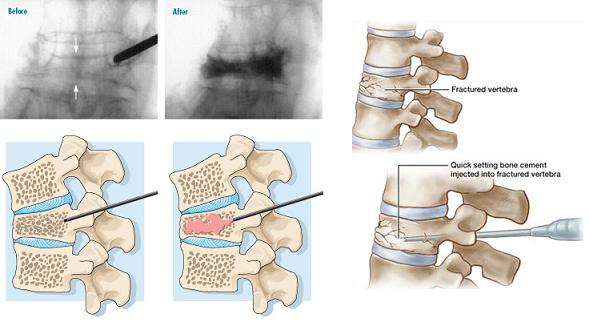
Disadvantages and Complications include: cement extravasation, nerve root compression –Radiculopathy, breaching the walls of the pedicle by the osteoplasty needle, prolonged fluoroscopic radiation exposure. A robot-guided vertebroplasty performed by the Mazor SpineAssist robot is shown in the figure below.
Phase I – Preoperative CT Scan: Trajectory planning and Intra-operative fluoro-CT registration (see figure below)
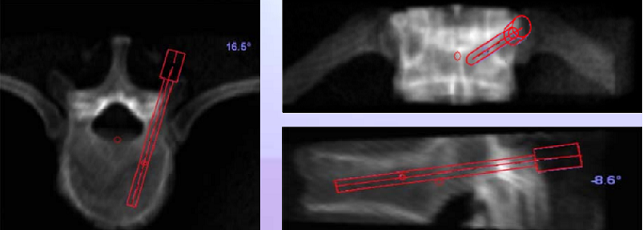
Phase II – Preoperative planning: The osteoplasty needle is placed on the CT-model, choosing ideal trajectories and length of the needle inserted (see figure below)
Phase III – T Frame Attachment: T-frame is mounted onto the patient spine, 2 Schantzpins into the PSIS, one K-Wire to the spinous process (see figure below)
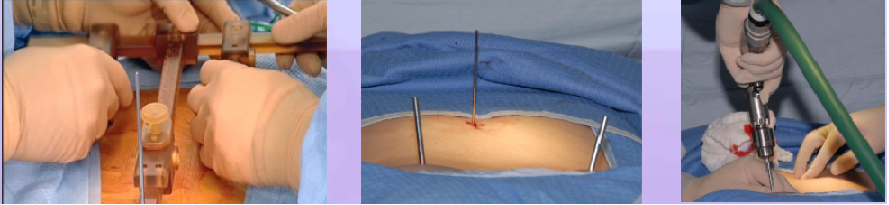
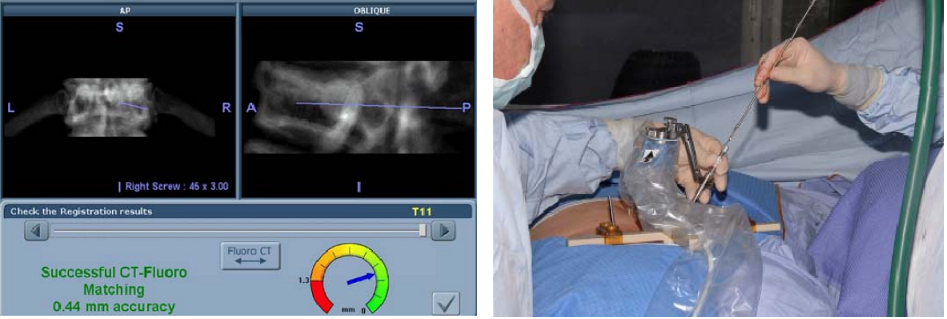
Phase IV – Automatic Registration: Pre-operative CT scan is matched to 2 intra-operative fluoroscopic images (AP & OBL) at the work station (left figure below)
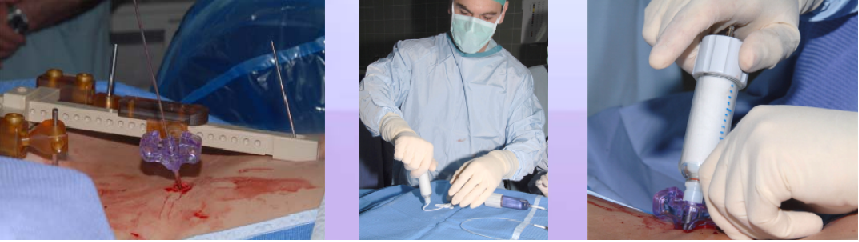
Phase V – Robotic guided drilling: Robotic device attached to T frame, software controls robot's motions, robot dictates entry point and trajectory according to the surgeon preoperative plan (right figure below)
Phase VI – Insertion of the cement: Osteoplasty needle is inserted through the K-Wire, cement insertion using fluoroscopy monitoring (see figure below)
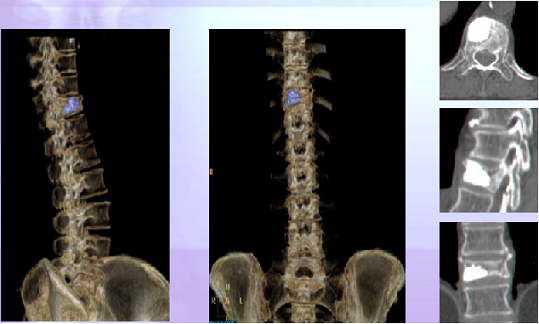
Post-operative CT Reconstruction
Advantages of Robot Assisted Vertebroplasty are: Additional indication – burst fractures, reduced radiation exposure time, low rate complication –can treat acute “fresh”fractures as analgesic procedure, reduced procedure time in cases of multiple fractures or in the need for biopsies, better accuracy –the new ZZEBRA partition
Innovations in Minimally Invasive Spinal Surgery
Recently, a new technology has been adopted in robot-assisted minimally invasive spinal surgery. Previous methods required extensive use of fluoroscopy and a large open vertebral exposure to visualize the pedicle of each vertebra for spinal fusions. Patients with spinal deformities and previous surgeries have strong indications for utilization of this technology.
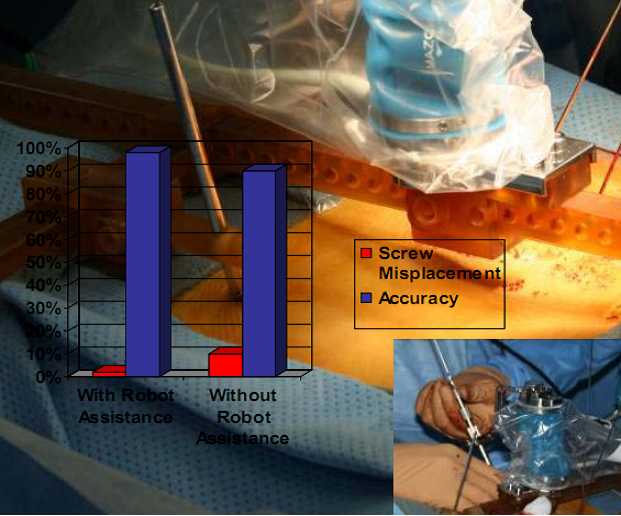
Historically, robotic devices were very large and cumbersome requiring valuable floor space in the surgical suite. With this new technology, the robotic assistive device is attached directly to the patient and remotely controlled by the surgeon using a computer workstation with a touch screen monitor. Preoperative Computerized Axial Tomography imagery and intra-operative fluoroscopic images are used to plan the operative approach. A virtual three dimensional image is created and the surgeon determines the length and placement of each pedicle, facet, or trans-laminar facet screw. Benefits of robotic assisted devices used for the treatment of spinal deformities include: decreased radiation exposure by decreasing the number of fluoroscopic images taken and increased accuracy of screw placement by 98%. Without the use of robot guided assistance, screw misplacement has occurred in over 10% of patients (see figure below). Placement of pedicle screws in patients with scoliosis or abnormal or missing anatomical landmarks from previous surgeries may have increased risks of neurological complications and thoracic deformities. The use of robotic assisted devices in minimally invasive spinal surgery has significantly decreased radiation exposure and improved the placement accuracy of each pedicle, facet, or trans-laminar facet screw thus decreasing overall patient recovery time.