|
|
| |
 Clinical Trial
Clinical Trial
|
|
|
|
| |
RAMP (Real-time Assessment of Myocardial Perfusion Imaging)-1 and -2 are Phase 3 trials conducted to determine if perflubutane polymer microspheres (PPM) injectable suspension (formerly AI-700), a synthetic biodegradable ultrasound contrast agent, can assess myocardial perfusion and detect coronary disease in patients being evaluated for inducible ischemia. RAMP-1 and RAMP-2 were international, multi-center Phase 3 clinical trials designed to demonstrate that Imagify Perfusion
Stress Echo at stress and rest is non-inferior to nuclear stress/rest testing in patients being evaluated for inducible ischemia. Non-inferiority and superiority analysis, compared to nuclear, was measured in three primary endpoints: accuracy, sensitivity
and specificity.
|
|
|
|
| |
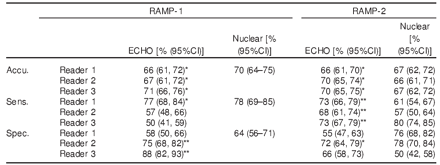
Among RAMP-1 and -2 angina patients from 28 international sites, those who underwent PPM echocardiography (ECHO) imaging (real-time and triggered) as well as 99mTc quantitative myocardial perfusion imaging (nuclear) at rest and at dipyridamole stress comprised the efficacy population (n=662). Images were interpreted for presence of defect (wall motion and/or perfusion) by independent blinded readers (3 ECHO and 1 - 3 nuclear readers for each study). Disease was defined by quantitative coronary angiography (= 70% stenosis), if available, or 90 day outcome with clinical history and nuclear assessment. Primary efficacy endpoints (accuracy followed by sensitivity and specificity) were evaluated using non-inferiority and superiority analysis (one-sided alpha=0.025) compared to nuclear. The median performing nuclear reader was the comparator in RAMP-2.
|
|
|
|
| |
RAMP-1 and -2 consisted of 285 patients (125 (44%) disease positive) and 377 patients (220 (58%) disease positive), respectively. Compared to nuclear, 6, 4 and 3 of 6 ECHO readers were non-inferior for accuracy, sensitivity and specificity, respectively. Moreover, 2 and 3 ECHO readers also demonstrated superiority for specificity and sensitivity, respectively (Table). Majority of AEs (headache, chest pain or discomfort, nausea and flushing) occurred following dipyridamole dosing, were mild, transient and required no treatment.
|
|
|
|
|
|
|
Table.3 Phase-3 Clinical Results
|
|
|
| |
RAMP-1 Results
-- Accuracy: 3 of 3 ultrasound blinded readers were non-inferior (p less than 0.004)
-- Sensitivity: 1 of 3 ultrasound blinded readers was non-inferior (p=0.002)
-- Specificity: 2 of 3 ultrasound blinded readers were superior (p less than 0.006)
|
|
|
|
| |
RAMP-2 Results
-- Accuracy: 3 of 3 ultrasound blinded readers were non-inferior (p less than 0.001)
-- Sensitivity: 3 of 3 ultrasound blinded readers were superior (p less than 0.002)
-- Specificity: 1 of 3 ultrasound blinded readers was non-inferior (p=0.013)
|
|
|
|
| |
PPM ECHO imaging has similar diagnostic performance compared to nuclear perfusion in chest pain patients being evaluated for inducible ischemia. In the RAMP-1 and -2 trials, Imagify was well-tolerated with the majority of adverse effects (AEs) reported being mild in intensity,
transient, and resolved without residual effects. The most common AEs reported were headache, chest pain or discomfort,
nausea and flushing and occurred following dipyridamole dosing (an alternative to exercise stress) and required no treatment.
|
|
|
|
|