| Valves based
on biological tissue were developed in the
1960’s. These
valves use porcine or
equine tissue harvested from the aortic root.
The valve tissue is then treated with Glutaraldehyde,
which sterilizes
the tissue to prevent an immune response.
It also induces the cross linking of collagen fibers,
strengthening the tissue. These
leaflets are then attached to a sewing
ring, with the final result closely resembling the structure and
mechanical
properties of a native heart valve.
As a result,
these valves mimic the natural blood flow
perfectly. Further,
the Glutaraldehyde
treatment makes the surface of the valve resistant to clots and
thrombus
formation. Therefore,
tissue valves are
recommended for individuals with bleeding disorders and others who
would otherwise
be able to take the anti-coagulant drugs required with mechanical valve
implantation.
There are
inherent drawbacks with tissue valves.
First, the implanted structure resembles the
native structure that failed in the first place.
As a result, the lifespan for a tissue valve
is much shorter than that of a mechanical valve.
Further, although Gluteraldehyde treatment
results in resistance to thrombus formation, it can increase risk of
calcification,
the buildup of calcium on the surface of the valve.
This can not only hinder the operation of the valve,
but also spread to the host's tissue as well.
Major manufactuerers of tissue valves include Edwards Lifesciences (see figure 8) and Medtronic.
|
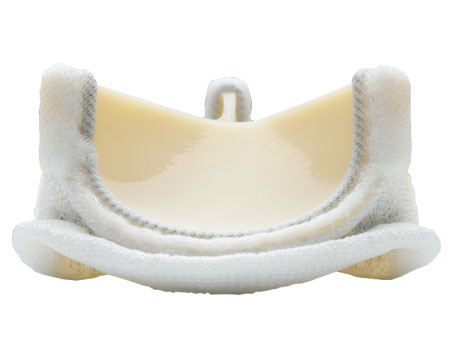
Figure 8: Edwards Lifesciences' PERIMOUNT Magna heart valve is made of porcine tissue. |
