Future Developments
|
Both mechanical and tissue heart valves have major
shortcomings that have yet to be addressed.
Applications of recently developed technologies to heart valves show
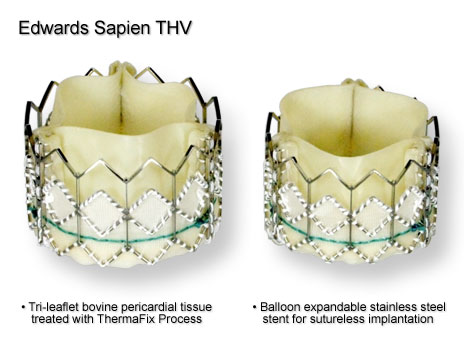
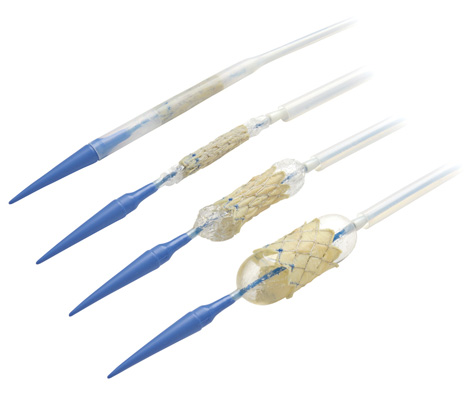
promise in addressing these shortcomings. Problems with thrombosis and clot formation are the major drawback to mechanical heart valves. Recent development in materials sciences of vitreous carbon, also known as glassy carbon, may address these problems. Compared to the current pyrolytic carbon that is used to coat the surface of mechanical valve leaflets, vitreous carbon is not as durable. However, it is less porous, preventing the deposition of protein and the ingrowth of blood vessels. This makes the leaflets less likely to become problematic. This development also has the potential to reduce or even eliminate the need for anti-thrombogenic medications in heart valve replacement patients. For tissue based valves, the majority of work has been done to develop transcatheter heart valves. The flexible properties or the leaflet are utilized to make a collapsible valve with a framework similar to that of a stent. This means that the valve can be inserted and placed using a catheter and guide wire system that is usually seen in other types of vascular surgery. Once in place, the valve is expanded into its final shape using a balloon catheter. Two products currently in development and awaiting clinical trail are the Edwards SAPIEN valve (see figure 9) and CoreValve Aortic Bioprosthesis. A third device, Medtronic's Melody (see figure 10), was recently approved for use in Canada and is intended for use in pediatric valve disorder cases. |
Previous BME 240 Home Next
Page design and content by Vincent Nguyen-Hoai