


Naturally-derived Scaffold Approach (Decellularized Tissues): Introduction
This technique relies on obtaining the proper tissue from a xenogenic or allogenic source, and using a series of detergents, enzyme inhibitors, and buffers that remove all cellular debris except for the ECM. This significantly reduces the immune response from the host, while allowing for the mechanical properties of the natural tissue to be relatively maintained. These decellularized tissues are normally implanted without any cells to allow for the host to infiltrate and invade the tissue. The true advantage of this technique lies in its ability to promote specific remodeling and regeneration by the host.

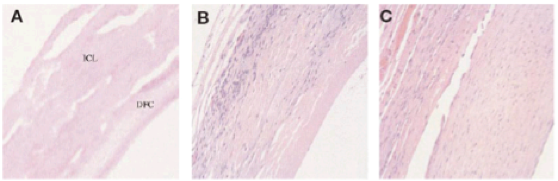
Figure 16. Histological sections of the graft before (a), 28 days (b), and 90 days (c).
Histology also revealed the presence of fibroblasts, SMCs, and a small inflammatory response within the graft. After 90 days, remodeling was observed throughout the entire cross-section of the graft, and the bDFC layer was almost completely remodeled. To confirm the presence of the SMCs within the graft, immunohistochemical analysis was performed to detect for α-smooth muscle actin. SMCs were indeed present in both 53 and 90 days post implantation explants, while the 90 day explant had a much more organized SMC network (Figure 17). This further confirms the observations that mechanical stimulation by the blood flow induces a more organized network of SMCs, which has been observed by many other groups.
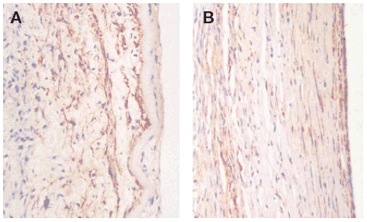
Figure 17. Cross-section of graft 53 days (a) and 90 days (b) post implantation. The 90 day had a much more organized network of SMCs.
To characterize the lumen of the graft, explants removed at 90 days were immunohistochemically analyzed to detect for any endothelialization by staining for CD31. The analysis revealed the presence of a monolayer of endothelial cells on the interface of the vessel and the lumen (Figure 18). The process of the neighboring endothelial cells migrating onto the graft still remains unclear, and is much more evident in animal models than human models. Scanning electron microscopy showed the arrangement of the endothelial cells, which followed the direction of blood flow.
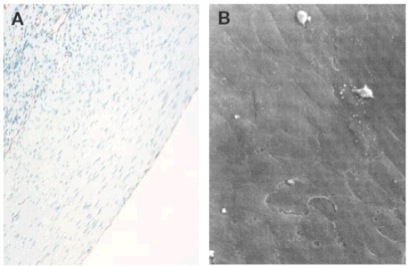
Figure 18. Immunohistochemical staining for CD31 (brown-purple, a) and SEM of the endothelium (b)
The endothelium was also tested for functionality by using a series of vasomotor reactive agents on a section of the graft. On 2-month explants, the grafts were subjected to various contractile agonists (norepinephrine, serotonin, bradykinin) and did respond with contraction of the graft. However, when using KCl as a contractile agent, the graft was able to generate a 2-10 fold less force than native vessels when exposed to the same agents. When exposed to acetylcholine, which is a vasodilator (endothelium dependent), the graft only relaxed by 16% of the precontracted tension at its maximum concentration. When exposed to nitroprusside, which is a relaxing agent independent of the endothelium, the graft completely relaxed. This showed that there was a high chance of incomplete coverage of the lumen with the endothelial cells, which resulted with a less-responsive relaxation when using acetylcholine vs. nitroprusside (Figure 19). This study opened up the possibility of remodeling, transanastamotic endothelialization, and rapid host infiltration using a naturally derived material from the small intestine.
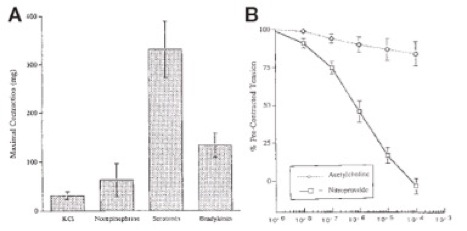
Figure 19. Vasomotor test results using various contractile agents (a), and amount of relaxation by acetylcholine and nitroprusside on the graft (b).
Limitations
This approach has several limitations that should be mentioned. First, the process of removing the submucosa of the small intestines is very tedious and requires specialists that can handle this material. Second, the thrombogenic properties of this material are still relatively unknown, and there are many aspects of this material that must be understood before clinical considerations. Third, there is a lot of variability when it comes to natural materials, so the mechanical properties of one sheet of SIS compared to another may be vastly different. Finally, the decellularization process may significantly impact the size of the tissues along with its mechanical properties; specifically, shrinkage and the reduction of the tissue’s ultimate tensile strength and compliance has been observed (Isenberg, 2006).
Procedure
The primary tissue source for this study was a porcine intestinal collagen layer (ICL) that was derived from the submucosa of the small intestines (SIS). The ICL was chemically treated to lyse the native cells followed by saline washes to remove the cell lysates. The remaining components of the ICL was primarily type I collagen, free of nucleic acids, lipids, and other proteins. The ICL was then exposed to several treatments to reduce thrombogenicity, wrapped around a Teflon-coated mandrel twice, and crosslinked together. The final step was impregnating the ICL with bovine dense fibrillar collagen (bDFC) to improve the patency of the graft.
Two specific mechanical tests were performed on this conduit after fabrication: suture retention strength and burst pressure. The suture retention strength of the conduit was two-fold higher than the surgical requirements (3.7 ± 0.5 N, n = 16), and the burst pressure was 931 ± 284 mmHg, n = 10), which was sufficient to withstand arterial pressures. Porosity was also quantified to ensure that there was sufficient space for cellular infiltration (4.5 ± 2.9 x 10-4 mL/cm2/min, n = 18). The grafts were subsequently removed at 28, 53, and 90 days post implantation.
Results
After 4 weeks of implantation, the graft was removed and histology was performed. The host cells infiltrated the ICL ECM and began the remodeling process by increasing wall thickness, while the lumen (bDFC layer) remained intact (Figure 16).
