


Biodegradable Scaffold Approach: Introduction
The general idea of this approach is to seed a particular cell type onto a degradable polymer scaffold that can support tissue growth and remodeling via proteolysis or hydrolysis. Cell seeding densities are usually below optimal cell distribution to allow for gradual breakdown of the polymer along with cellular growth, ECM deposition, and host invasion. The advantages to this technique over other natural material approaches are two-fold. First, important parameters such as mechanical properties and resportion rates can be tuned to optimize tissue growth via chemical manipulation of the polymers. Second, the polymer material provides the initial mechanical support cells require until they can secret their own ECM (Isenberg, 2006).


Figure 12. Constructed graft soaking in saline for 1-week.
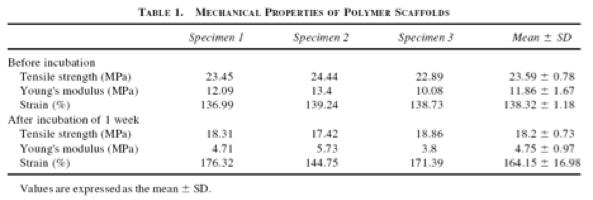
Table 2. Mechanical properties of grafts before and after incubation.
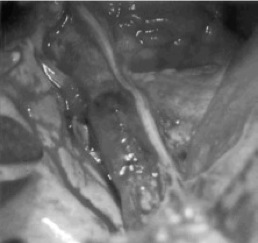
Figure 13. Graft implanted as vena cava replacement.
Results
Angiograms were performed at 3, 4, 5, and 6 months post implantation. By 3 months, the scaffold completely degraded, while the graft remained patent. The longest time period a graft remained patent was 13 months (n = 1) with virtually no indications of stenosis via angiography (Figure 14). Histological analysis revealed no remnants of the polymer scaffolds while the composition of the grafts were collagen fibers, organized elastic fibers, and cellular components (Figure 15).
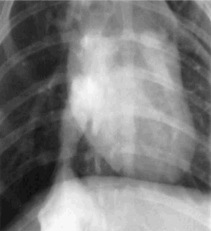
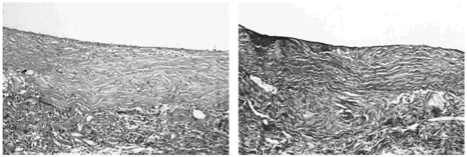
Figure 14. Angiogram of integrated graft on the inferior vena cava.
Figure 15. Histology revealed almost no remnants of the polymer scaffold while collagen fibers, organized elastin, and cellular components invaded the graft.
Endothelialization of the graft was apparent after in vivo implantation. Immunohistochemical staining for factor VIII revealed the existence of a lining of endothelial cells on the luminal surface at 3, 5, and 6 months post implantation. Immunohistochemical staining for α-smooth muscle actin and Desmin, which are common proteins expressed by SMCs, revealed the distribution of SMCs around the endothelial layer (Watanabe, 2001) .
Another group utilized a similar technique to be the first ever to successfully use a TEVG to repair an occluded pulmonary artery in a 4-year old child. The graft was fabricated in a similar fashion (with an autologous cell source from a peripheral vein from the patient, which were seeded into the polymer scaffold). There were no signs of stenosis or aneurysm formation on the grafter after 7 months (Shin’oka, 2001).
Limitations
The conditions in which these polymer scaffolds are fabricated are toxic for cells, so seeding cells before the finished product may not be done. Also, premature implantation of scaffolds carries a major risk because if there is enough ECM deposited by the cells before the polymer completely degrades, the graft would not be able to function properly. Biocompatibility is also another issue because there is only a handful of polymers that have been FDA-approved for clinical use, and the approval process of a new polymer is a very long, drawn out process that requires extensive tests in both animal and human models.
Procedure
The polymeric scaffold that was used in this study was a hybrid material that was composed of PGA sheets combined with polycarpolactone-co-polylactic acid copolymer P(CL/LA, which degraded via hydrolysis. Using a mixed autologous cell population (primarily vascular myofibroblasts and vascular SMCs) from the femoral veins of each canine, the scaffold was seeded and cultured for a week. Mechanical testing was performed before and after incubation of the graft in normal saline at 37° for 1 week to ensure sufficient tensile strength (Table 2, Figure 12). After the 1-week culture period, the graft was implanted into the same dog’s inferior vena cava for in vivo studies (Figure 13).
