


Sheet-based Approach (No scaffold used): Introduction
The main principle of the sheet-based or the “self-assembly” technique is to develop sheets of cells that fuse together to create a tubular structure for the vascular graft. This approach does not employ the typical tissue engineering technique of cell transplantation, which involves seeding a certain cell line into an extracellular matrix (ECM) that is fabricated separately. Instead, this technique relies on the cells to secrete their own ECM, which will eventually bond the cells together to form a sheet. Auger et al, used this technique and was able to produce the most impressive, completely-biological graft up-to-date (Isenberg, 2006)


Table 1. Summary of mechanical properties of the graft and its intended replacements.
A long term study was performed in nude rats with the TEVG as abdominal interpositional grafts. After 90 and 225 days, the graft was removed, and had completely incorporated into the surrounding tissue. Before the removal, Doppler-ultrasound imaging revealed no mechanical failures and an intact luminal diameter. After the removal, the explant retained a smooth lumen, intact anastamoses with no blood infiltration into the graft wall, and did not display any signs of stenosis or aneurysm formation (Figure 10). The overall patency was 85% with no post-operative therapy to prevent platelet adhesion.
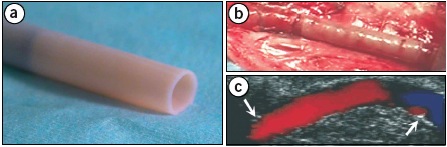
Figure 9. Finished TEVG (a), implanted into the femoral artery in the canine model (b), and Doppler-ultrasound image showing flow through the graft (c).
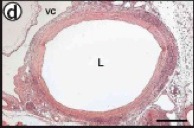
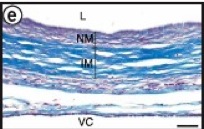
Figure 10. Histology revealed an intact, open lumen with distinct layers formed on the graft.
Primate implantation studies were also performed (n = 3) for 8 weeks with the TEVG as interpositional arterial grafts (immunosuppressed macaques) (Figure 11a). Similar to the immunosuppressed rat study, the graft displayed mechanical stability, a smooth lumen, and intact anastamoses throughout the study (Figure 11c, g). On the other hand, histology on the sections of the explant revealed a moderate immune response to the human tissue with leukocytes penetrating the luminal wall. In both rat and primate cases, remodeling was apparent in these grafts, which was based on the formation of α-actin positive cells on the interface between the adventitia and the internal membrane layer. The patency of the graft also remained relatively constant after 5 weeks in vivo (Figure 11h).
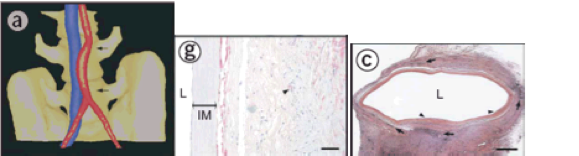
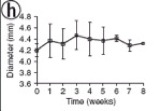
Figure 11. Position of the graft (a), histological sections of the graft (c, g), and the patency of the graft through the 8-week study (h).
Through in vivo studies, histological analysis, and ultrasound imaging, the TEVGs using the sheet-based approaches proved to be successful in rat, canine, and primate models (L’Heureux, 1998).
Limitations
The main limitation in this technique is that these types of grafts are significantly less compliant than native-small caliber vessels, which could lead to various complications such as intimal hyperplasia due to compliance mismatch. The lack of compliance in these grafts has been determined to be caused by the lack of sufficient elastic fiber networks, but recent studies have been able to induce elastogenesis.

Procedure
Human skin fibroblasts were cultured in culture flasks with promoters that induced ECM deposition, which ultimately resulted with a cohesive sheet that could detach from the flask. In the final product, the graft consisted of three distinct layers: a living adventitia, a decellularized internal membrane, and a functional endothelium. These sheets were continuously grown for up to 15 weeks, and had an increasing thickness rate of 5 µm/week. The time period for the formation of a full intact graft was ~28 weeks. The internal membrane was assembled by wrapping an 8-week old fibroblast sheet around a Teflon-coated stainless steel tube (mandrel) up to 3 revolutions. After a minimum of 10 weeks for maturation the layers of the wrapped sheet fused together and formed a homogeneous tissue. This fused sheet was then dehydrated to decellularize the membrane to allow for endothelial cell seeding. The living adventitia was formed using a similar technique, which also involved wrapping the fibroblast sheet around the mandrel, removing the fused tissue after its second maturation phase, and finally seeding the autologous endothelial cells from each patient into the lumen of the grafts. The grafts were then subjected to mechanical stimulation with pulsatile flow from 3 mL/min to 150 mL/min over a 3-day preconditioning period.
Results
Several important mechanical test were performed to test for parameters such as burst pressure, compliance, and suture retention strength. The graft had an internal diameter of 4.2 mm, and had mechanical properties similar to the saphenous veins in a xenografted canine model with immunosuppressants (Table 1). The graft replaced a section of the femoral artery, and Doppler-ultrasound imaging revealed that vessel patency was maintained and flow remained uniform through the 2-week study (Figure 9). For the canine models, the autologous canine endothelial cells were extracted and used to form the endothelium of the graft.