BUILDING BETTER BREASTS
Stem Cell Techniques in Breast Augmentation
Why Bigger Breasts?
Tissue Engineering
Stem Cells & Breasts
Future Directions
References
Tissue Engineering
Stem cells are cells with special characteristics which make them ideal for regenerative medicine (and cosmetic surgery in certain cases). They have unlimited proliferation potential (they will not undergo senescence, or the inability to reproduce and subsequent apoptosis). This means that from a small culture of stem cells, in theory, a huge number of cells can be created and subsequently used in vivo. Embryonic stem cells have the ability to differentiate into any tissue in the body (pluripotency) and therefore are useful for regenerative medicine because any tissue can be grown from them. Adult stem cells have a similar ability but their differentiation potential is more limited.
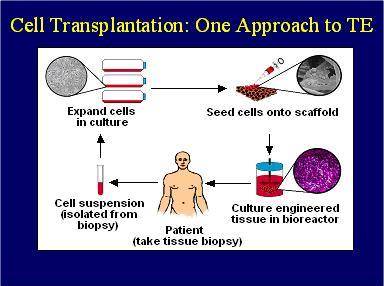
The cells are not the only important aspect of stem cell development, however. The cells exist in a scaffold known as the extracellar matrix, which, among other things, provides an attachment point which cells can adhere to in a three dimensional environment. The extracellular matrix also sends signals to the cells based on how the cells are adhered to the matrix, what the matrix itself is made of (collagen, fibronectin, etc.) and what other cells are present in the matrix. Other cells in the matrix can secrete growth factors and other molecules which can influence stem cell fate and are very important in differentiation of the cells in stem cell procedures. Stem cells often need to be seeded onto a scaffold as shown in figure 5 [7]. Scaffold can be synthetic polymers (like PLGA) natural materials (collagen) or they can be cultured with other cells who already have a scaffold, as in the technique discussed later on the site.
Stem cells have been cultured from many areas of the adult body. Bone marrow has been extensively investigated and is the most well characterized stem-cell source in the adult body, but recently adipose tissue has become an important source of stem cells as well. There are more stem cells in adipose tissue and these have greater proliferation potential than those derived from bone marrow[1] which means easier isolation and growth of adipose-derived stem cells than those of bone marrow. Also, adipose-derived stem cells need not be harvested as invasively as bone because fat is stored just beneath the skin, while bone marrow is found at the core of bones, requiring painful drilling and harvesting procedures. Finally, when harvested from adipose tissue, clinically relevant numbers of stem cells can be harvested from the body directly, circumventing the need for in vitro culture. Adipose derived stem cells have many possible lineages and are therefore very versatile. Nearly as versatile as bone marrow stem cells, adipose derived cells have been shown to differentiate into bone, fat, and other tissues [3],[4].
Cell and organ transplantation is not as simple as growing a new organ and throwing it into the body or injecting a lump of cells. The cells require something other than each other to survive: a blood supply, just like every other cell in the body. Without a blood supply the cells will die in culture or when injected into the body. Many growth factors, including VEGF and angiopoetin-1 are involved in the vascularization of a tissue construct.