Future challenges
Challenges on the differentiation process and how tissue engineering might help
Some studies focused on finding new sources of insulin producing cells besides human donors. Significant achievements have been claimed in the differentiation of insulin producing cells from stem cells. The stem cells could be embryonic stem cells, adult stem cells or induced pluripotent stem cells. Tissue engineering could help the differentiation process in many ways. Biomaterial scaffolds can be developed as cell carriers providing mechanical support.synthetic micro environments can be used to tightly control the fate of stem cells.
Mouse, monkey and human embryonic stem cells have been reported to differentiate into insulin-positive cells.( Mayhew, 2010)
The transplantation of insulin-producing β-cells derived from human embryonic stem cells and induced pluripotent stem cells (PSCs) holds great promise for therapy of diabetes mellitus. Although several protocols to generate glucose-responsive pancreatic β-cells in vitro have been described, the most successful approaches are those that most closely mimic embryonic development of the endocrine pancreas. Until recently, cells generated by these methods have exhibited immature pancreatic endocrine phenotypes. However, protocols that generate more functional β-like cells have now been described. In addition, small molecules are being used to improve protocols to direct differentiation of PSCs into endoderm and pancreatic lineages.
Generally, generating functional β-cells from human PSCs is achievable. However, there are aspects of β-cell development that are not well understood and are hampering generation of PSC-derived β-cells. In particular, the signaling pathways that instruct endocrine progenitor cells to differentiate into mature and functional β-cells are poorly understood. Other significant obstacles remain, including the need for safe and cost-effective differentiation methods for large-scale generation of transplantation quality β-cells, methods to prevent immune rejection of grafted tissues, and amelioration of the risks of tumorigenesis.
Beta cell neogenesis has been reported in many experimental conditions, supporting the presence of adult stem cells. Studies of particular interest are generation of insulin-producing cells from human adult duct cells. (Bonner-Weir, 2000)
The digested pancreatic tissue that is normally discarded from human islet isolations was cultured under conditions that allowed expansion of the ductal cells as a monolayer whereupon the cells were overlaid with a thin layer of Matrigel. With this manipulation, the monolayer of epithelial cells formed three-dimensional structures of ductal cysts from which 50-to 150- micrometer diameter islet-like clusters of pancreatic endocrine cells budded. Over 3-4 weeks culture the insulin content per flask increased 10- to 15-fold as the DNA content increased up to 7-fold. The cultivated human islet buds were shown by immunofluorescence to consist of cytokeratin 19-positive duct cells and hormone-positive islet cells. Insulin secretion studies were done over 24 h in culture. Compared with their basal secretion at 5 mM glucose, cysts/cultivated human islet buds exposed to stimulatory 20 mM glucose had a 2.3-fold increase in secreted insulin. Thus, duct tissue from human pancreas can be expanded in culture and then be directed to differentiate into glucose responsive islet tissue in vitro.
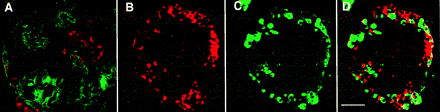
Double immunostained sections of cultivated human islet buds (CHIBs). (A) Cytokeratin 19 (FITC, green)-positive duct cells make up most of this CHIB with insulin-positive cells (Texas red) in several islet buds. Another CHIB shown with cell positive for insulin (red in B and D) and for the non-β cell hormones (glucagon, somatostatin, and pancreatic polypeptide) (green in C and D); D is the overlay of these red and green images. There are a few cells that coexpress both β and non-β cell hormones (yellow in D), indicating that some of the cells are immature and still in differentiation. (Magnification bar = 50 μm.)
Insulin-producing cells have been generated from iPS cells too. (Zhang, 2009)
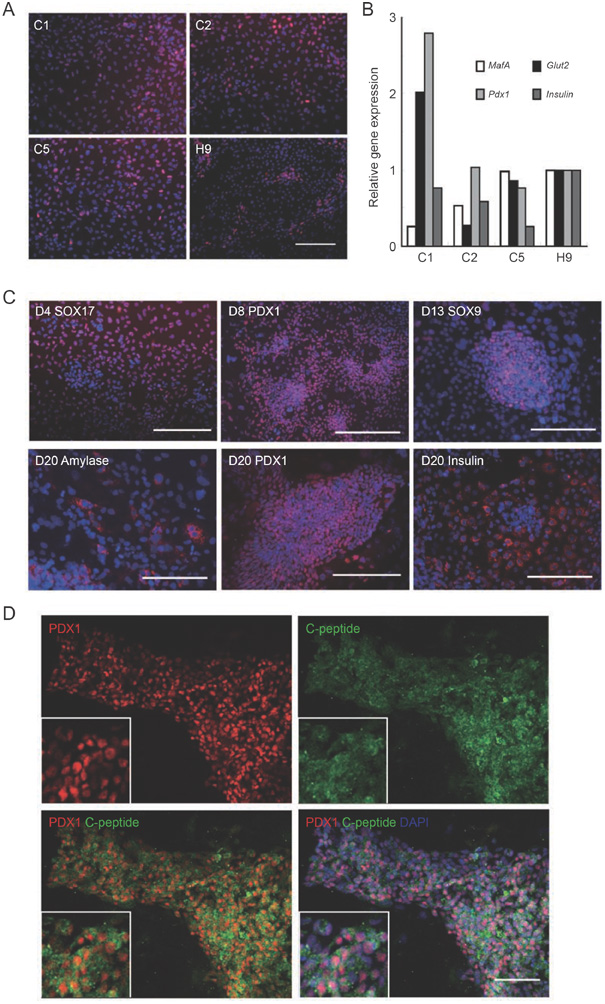
Human pluripotent stem cells represent a potentially unlimited source of functional pancreatic endocrine lineage cells. The human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells are used to differentiate into mature insulin-producing cells in a chemical-defined culture system. The differentiated human ES cells obtained by this approach comprised nearly 25% insulin-positive cells as assayed by flow cytometry analysis, which released insulin/C-peptide in response to glucose stimuli in a manner comparable to that of adult human islets. Most of these insulin-producing cells co-expressed mature beta cell-specific markers such as NKX6-1 and PDX1, indicating a similar gene expression pattern to adult islet beta cells in vivo.
 m.
m.