Recent advances
Transplantation of islets into the portal vein of diabetic patients has emerged as a promising procedure for the treatment of type 1 diabetes. However, shortages of donors and adverse effects leading to graft impairment and/or rejection have prevented this procedure from achieving widespread clinical application. Tissue engineering could be applied to solve the problem from different angles:
Tissue engineering can be used to help expanding islets in vitro before transplantation .
One study developed a tissue culture platform whereby isolated adult human pancreatic islets form proliferative duct-like structures expressing ductal and progenitor markers. Short exposure to islet neogenesis-associated protein (INGAP) induces these structures to reform islet-like structures that resemble freshly isolated islets (Hanley, 2009).
Tissue engineering can be used to help maintaining islets in vivo after transplantation.
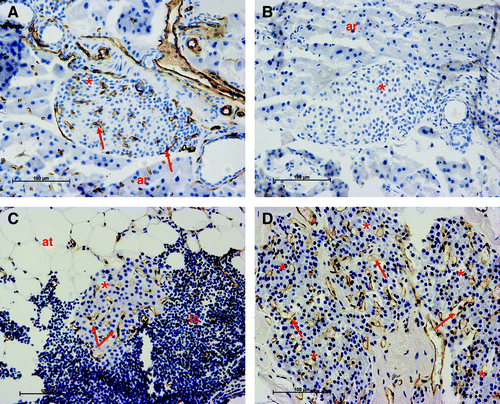
One study developed a method that could support the survival and function of transplanted islets using a prevascularized tissue engineering chamber (Hussey, 2009). In their studies, islets were transplanted into tissue engineering chambers established on the epigastric pedicle in the groin of diabetic mice. Islets were transplanted at the time of chamber implantation or with 21 days prevascularization of the chamber. Transplantation of islets into prevascularized chambers into diabetic RIP-Kb mice resulted in a significant reduction in blood glucose levels that became evident in the third week and improved glycemic control as measured by a glucose tolerance test. This study highlights that islet survival and function are potentiated by allowing a period of prevascularization within tissue engineering chambers before islet transplantation. This novel prevascularized chamber may be an improved method of islet transplantation. It can be easily accessed for islet seeding, easily retrieved, and transplanted to alternative anatomical sites by microvascular methods (Hussey, 2009).

Surviving islets immunostained for blood vessels with PECAM-1 in (A) nondiabetic control pancreas;(B) nondiabetic pancreas, negative control (C) an islet stained for blood vessels transplanted with immediate seeding from a chamber graft 12 weeks after transplantation; (D) a mass of five islets immunostained for blood vessels transplanted with delayed seeding from a chamber graft 12 weeks after transplantation. Islets (*); inflammatory cells (ic); blood vessels (arrows); adipose tissue (at); acinar tissue (ar).