Background
Previous tissue engineering scaffold-based approaches have aimed to induce tissue self-assembly in applications such as cell-sheet engineering, centrifugal casting and magnetically driven approaches. Manipulating the cellular environment (i.e. surface tension, restricted geometry etc) may stimulate cells to self-aggregate to desirable geometrical shapes or migrate such as is done in rotating-culture systems and hanging-drop method. These concepts have been extended to other areas of research; utilizing microfluidics, for example, for the high throughput generation and screening of compact spheroids and cell encapsulation. The emphasis of understanding the process by which cells self-assemble to 3D constructs has continued to grow to be central to comprehending morphogenesis and the development of living tissues. Evolving from tissue engineering, self-assembly processes have reached to other areas of research with increasing speed.
Fundamentally, bioprinting emphasizes the use of computer-aided technology and principles such as tissue self-assembly, synthesis and remodeling. Marketing an idea of "directed tissue self-assembly", organ printing utilizes these rapid prototyping technologies and biological principles to influence tissue development. Computer-aided design enables the precise and controlled placement and layer-by-layer deposition (also known as solid free-form fabrication) of cells, cell aggregates, cell-encapsulated hydrogels or any biologically related complex 3D structure along with other important chemical components such as nutrients and growth factors. With the simultaneous delivery of these cells, the natural autonomous organization of cells may potentially lead to the controlled patterning and assembly of complex 3D constructs.
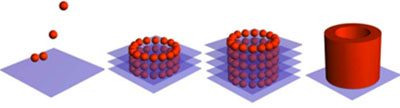 Bioink deposited from bioprinter onto hydrogel biopaper. Alternating layers of printed ring structure and gel (acts like a matrix) eventually form a smooth cylinder after gel relaxes and layers merge.
Bioink deposited from bioprinter onto hydrogel biopaper. Alternating layers of printed ring structure and gel (acts like a matrix) eventually form a smooth cylinder after gel relaxes and layers merge.Theoretically, bioprinting technology consists of six essential components: A CAD drawing of the desired engineered organ (blueprint); cells, cell aggregates or cell-encapsulated hydrogels capable of natural self-assembly (bioink); robotic-aided device for delivering the bioink (bioprinter); a container enclosed of the material to be deposited (biocartridge); a bioprocessible biomimetic hydrogel to transfer material on (biopaper); and a perfused container containing the resulting printed 3D tissue construct capable of post-conditioning (bioreactor). While only in the early stages of development, bioprinting has many potential applications and can be divided into three steps: pre-processing, processing and post-processing.
TOP

