Clinical results have been encouraging [3]. The first human trials by the Melman group in 2006 had 11 participants and was intended to demonstrate the safety of the gene therapy product. 6 of them had severe ED according to their IIED scores were injected with a low dose (500-1000 micrograms) of hMaxi-K while another three were injected at a higher dose of 5000 micrograms and two with 7500 micrograms. IIED is an International Index of Erectile Function with the severity of ED
classified into five grades based on the
six-item erectile function (EF) domain, i.e.
severe (score 0 –10) to a normal score (26-30) [7]. Figure 4 shows the demographic breakdown of the participants.
None of the participants recorded any adverse immune reaction proving the safety of the non-viral vector method used to transfect the genes. There was also no germline transmission of the recombinant genes. However the efficacy could not be determined reliably owing to the very low doses and small sample size, although at least one subject in each of the higher dose group reported and increase in frequency and duration of erection.
However trials in rats [2] carried out by the same group reported a dramatic increase in the number and duration of erections after gene transfer of the hMaxi-K plasmids,owing to restored intra cavernosa arterial pressure (Figure 5).
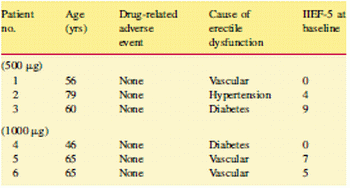
Figure 4: Patient demographics and results in the Phase I trial for hMaxi-K gene transfer treatment treatment
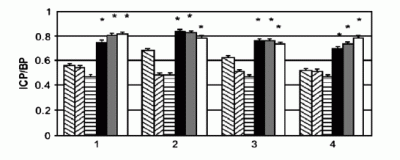
Figure 5: Results of a study in rats on the effect of hMaxi-K plasmid injection in restoring intra cavernosa pressure (ICP) relative to normal blood pressure (BP). The hatched bars are control or low dose groups, the solid black,gray and white bars are groups that received high dosages of hMaxi-K plasmid
|
