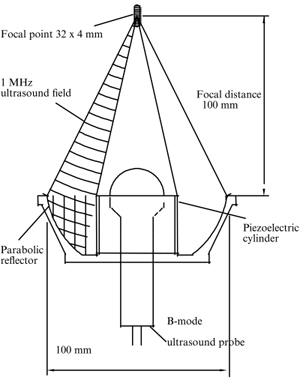
The most important aspect of HIFU instrumentation is the ultrasonic transducer that generates pressure waves at ultrasonic frequencies of around 2-4 MHz. The most popular transducer used is spherical lead zirconate titanate (PZT) which is a piezo-ceramic material [7]. Piezo-composite materials are also used as transducers and these consist of diced ceramic filled with a polymer. The ultrasonic beam generated is focused by a parabolic reflector and by changing the properties of the reflector and transducer, the focal length of the beam can be altered. The transducers receive between 100-500 W of power and can generate a maximum pressure of 2-4 MPa. Laboratory based HIFU instruments have beam focal lengths in the 10s of centimeter range while clinical instruments have shorter focal lengths around 3-5 cm since they are held up to the skin during use. Ultrasound beam intensities range from 1500-3000 W / sq. cm [8]. Methods of altering the instrumentation for large treatment areas include mechanically blurring the focus so as to broaden the treatment area and using arrays of ultrasound beams. A schematic of the transducer is included at right [7]. |
 |
A clinical HIFU unit consists of a control console, power generator, cooling system, and a probe that has standard ultrasound imaging capability as well as the high intensity focused ultrasound head. There are currently two commercially available clinical HIFU instruments: Ablatherm, by EDAP TMS, and Sonablate manufactured by Focus Surgery Inc.
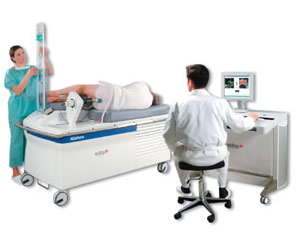
The Ablatherm system, developed by the French company EDAP TMS, includes a treatment module, a control table with computer, and a diagnostic ultrasound device included alongside the treatment module. The treatment module is motorized and computer-driven, allowing for movement of the module head in three dimensions. The Ablatherm probe has a 4 cm focal length driven at 2.25-3 MHz and has an intensity of 1000 W/ sq. cm. Unique to the Abltherm is that it has a separate transducer for imaging, capable of frequencies up to 7.5 MHz. This system uses endorectal insertion for entry into the body and allows for visualization and treatment of the prostate. After insertion, ultrasound images are first recorded and stored in the computer. The treatment head can then automatically move to each spot stored as a lesion and deliver its ultrasound therapy. The Ablatherm system has carried the CE mark since 2000 after successful demonstration of its efficacy treating prostate cancer in a clinical trial of the instrument and is currently approved for use in Europe, South Korea, Canada, and Russia. |
|
|
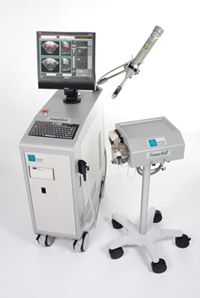
The Sonablate system, developed by Focus Surgery of Indianapolis, Indiana, is a mobile instrument consisting of a chiller, probe, and console. The chiller circulates and cools water flowing around the HIFU transducer in order to cool the probe. The probe itself is also motorized, allowing for computer-driven movement and also includes both ultrasound imaging capability and HIFU treatment. The console contains the subsystem for the ultrasound imaging system and support components for the HIFU probe, in addition to a computer with a custom graphical user interface for treatment planning and execution. The Sonablate system probe uses a 4 MHz PZT transducer that produces a beam of 3, 3.5, or 4 cm focal length with an intensity at the focus of 1680-2000 W/ sq. cm. As of May 2005, the Sonablate system had completed phase I clinical trials in the US and had been granted an investigational device exemption by the FDA to begin phase II and III clinical trials. While the phase III study has started, as of this writing patients are not currently being enrolled according to the company website. The Sonablate system is currently approved for use in essentially all of Europe, Asia (China, Japan, Iran), South America (Argentina), Africa (South Africa), Austrialia, and in North America (Canada, Mexico, Costa Rica, Dominican Republic). |
Created by Austin Moy
BME 240: Intro to Clinical Medicine
Biomedical Engineering
University of California, Irvine
Last Updated:
June 11, 2008