|
|||||||||||||||
|
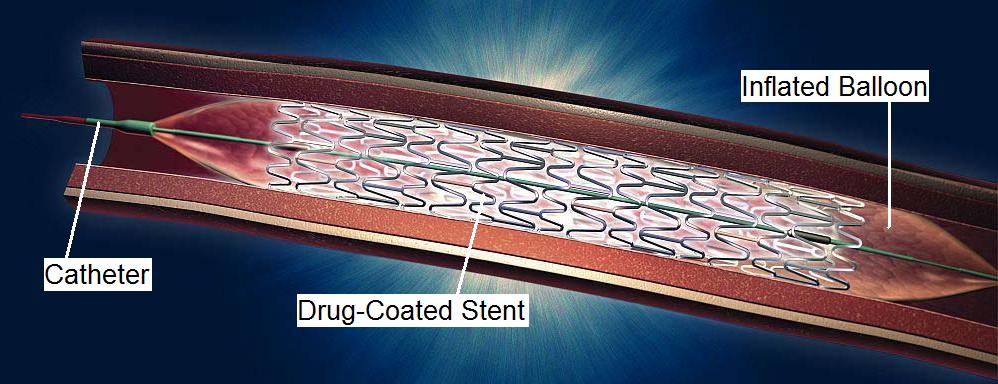
Current DevicesThis section discusses which drug-eluting stents are currently out in the market, prefaced by a brief overview of how drug-eluting stents work. How Drug-Eluting Stents WorkDrug-eluting stents are comprised of three main components: the stent, the drug, and the carrier. The metal stent is the expandable metal framework that is implanted in the diseased artery. The stent is coated with a drug that prevents reocclusion in the artery. The carrier is typically a polymer which controls the slow release of the drug to prevent restenosis. A diagram of a typical stent is shown below (Figure 4).
The placement of drug-eluting stents in the location of the diseased artery comprises of several main steps. The collapsed stent is mounted onto the end of a balloon catheter. The catheter is inserted through a femoral artery and threaded to the site of bloackge. The balloon is then inflated, which causes the lumen of the artery to widen and the stent to expand. The balloon and catheter are then withdrawn, leaving behind the wire stent. Within a few weeks after the placement of the stent, the endothelium (inner layer of the artery) begins to grow over the stent. The carrier controls the release of the drug from the stent over a period of several months. There are several factors that the cardiologist must consider when choosing the appropriate drug-eluting stent. The length and diameter of the stent must match the size of the artery in which the stent will be implanted. The cardiologist must also ensure sufficient deployment of the stent, making sure that it is fully expanded once in the artery. Current Drug-Eluting Stents on the MarketThere are currently two companies in the US market for drug-eluting stents, Johnson & Johnson (which uses the drug sirolimus) and Boston Scientific (which uses the drug paclitaxel). Johnson & Johnson (Cordis Corporation) Cordis Corporation, under Johnson & Johnson, released the first drug-eluting stent, FDA approved in April 2003. Their drug-eluting stent is called "Cypher", which uses the drug sirolimus. The Cypher stent is made of stainless steel and uses a polymer to elute sirolimus. Sirolimus is a relatively new immunosuppressant and antiproliferative drug, which was originally desgined to prevent rejection in organ transplantation. Sirolimus works by inhibiting the response to IL-2, which therefore blocks activation of T- and B-cells. This drug is used in the Cypher stent to reduce restenosis of a treated coronary artery. Boston Scientific Boston Scientific was next to release a drug-eluting stent, FDA approved in March 2004. Their drug-eluting stent is called "Taxus", which uses the drug paclitaxel. The Taxus stent uses a polymer called Translute, also known as poly(styrene-bisobutylene-b-styrene) (SIBS) to release the drug. Paclitaxel is an antiproliferative drug that was originally developed for various types of cancer. Paclitaxel works by interfering with the normal function of microtubule growth, which prevents the growth and spread of tumorous cells. There has also been much clinical success in paclitaxel reducing restenosis rates. Other Companies Although Johnson & Johnson and Boston Scientific are currently the only two companies in the US market with FDA-approved drug-eluting stents, they should be wary of other competitors who will be entering the market soon. Medtronic is expected to get FDA approval in late 2006. Abbott Laboratories, which recently purchased Guidant, is expected to get FDA approval for their drug-eluting stent in early 2007. Medtronic will soon be releasing its drug-eluting stent called "Endeavor", which uses the drug zotarolimus (or ABT-578, a drug designed by Abbott Labs). The Endeavor stent uses a polymer called phosphorylcholine to mimic the structure of a cell membrane, which leads to a faster healing response time and less inflammation. The Endeavor has so far proved to have excellent clinical outcomes in clinical effectiveness and safety, and Medtronic expects FDA approval in late 2006 or early 2007. Abbott Labs has its own drug-eluting coronary stent system known as "Xience", which uses the drug everolimus. Originally, Abbott was planning to release its "Zomaxx" stent that releases zotarolimus, but canceled its development in Fall 2006. It decided to go with the Xience stent, due to the acquisition of Guidant. The Xience stent uses Abbott's TriMaxx stainless steel and tantalum stent to elute everolimus. Abbott Vascular is also working on developing bioresorbable drug-eluting stents and stents that release a combination of drugs. Next: Future Research
|
Home![]() Clinical
Background
Clinical
Background![]() Current Devices
Current Devices![]() Future
Research
Future
Research![]() References
References
Website by
Ken Cheng![]() UC-Irvine, BME240
UC-Irvine, BME240![]() This site was last updated
06/12/07
This site was last updated
06/12/07