|
Future Work
The work that is being conducted has shown very
promising results. However, as pointed out
before, it is difficult to find peptides that are
specific to every cancer. Since cancers
can be inherently different from each other and
possess no common unique markers that can be
targeted, it can be a long process in finding homing
peptides to target every cancer. Also, due to
the fact that cancers evolve, homing peptides found
today could be useless at the time they reach
clinical trials. Cancers have been known to
change their surface antigen expression to avoid
recognition by the host immune system. The key
to work around these evasion strategies is to target
a surface antigen that is integral to the tumor's
viability. The success of this therapy is
dependent on technologies that can create a high
throughput method to detect surface peptides and
then create a complementary homing peptide to it.
Much of the newer research is focused on targeting
the vasculature of tumors and preventing
angiogenesis from occurring. It is known that
cancers can live in hypoxic environments for periods
of time so delivery devices must be able to sustain
their anti-angiogenetic abilities to prevent the
tumor from winning the battle of attrition. To
sustain the homing peptide/drug conjugate in vivo
it must be attached to a carrier. Nanocrystals
need to be fabricated in a way to increase the
incorporation of the drug within the tumor cell
while preventing incorporation into normal cells or
uptake by the reticuloendothelial system. Work
has already show that coating the surface of
nanocrystals with polyethylene glycol reduces the
amount of nanoparticles removed from circulation by
the spleen and the liver.
In order for these tests to proceed to any type of
clinical trials, drug toxicity tests need to be
performed on the wide range of chemotherapeutic
drugs that will be used. Although the intent
is to target the drugs to the tumor, byproducts of
the drugs can lead to acute toxicity.
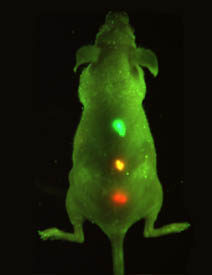
Simultaneous
multi-colored imaging of quantum-dot tagged cancer
cells. The colors are visible through the skin of
the live mouse when illuminated with a black light.
Courtesy Shuming Nie, Ph.D., Georgia Institute of
Technology.
|