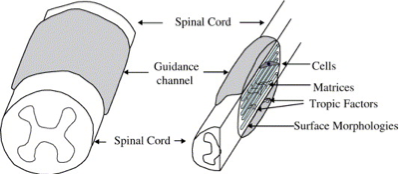
Strategies to facilitate CNS repair
Owing to the profound impact of CNS damage, extensive studies have been carried out aimed at facilitating CNS repair following injury. Many strategies have been developed to facilitate axonal reinnervation and to direct their outgrowth. A variety of bridging devices, which use synthetic or biological substrates, are being developed such as biomaterial bridges, peripheral nerve grafts, acellular muscle grafts, blood vessels, and fetal tissue grafts. In addition, many approaches to suppress CNS inflammatory response or to promote regeneration at the lesion site are being investigated in conjunction with bridging techniques. These approaches include disinhibition using antibodies to block inhibitory molecules, administration of axon!
al outgrowth-promoting molecules, co-transplantation with normal cells such as Schwann cells, immature astrocytes, macrophages, olfactory ensheathing cells, stem cells and precursors, or genetically engineered cells \, and application of x-irradiation and electrical and/or magnetic stimulation. Huang et al. developed the therapeutic vaccine approach by stimulating the animal's own immune system to generate antibodies against all of the spinal-cord-derived growth inhibitory proteins with a unique immunization technique. Utilizing this approach, regeneration of corticospinal tract fibers for a long distance was observed in mice. Another approach, in which antibodies were produced against CNS inhibitory proteins by passive immunization of the animal at the time of lesion, appears promising in encouraging axonal growth during the time interval of the antibodies entering into the CNS lesion site which occurred immediately following the injury, and the complete formation of the g!
lial scar at a later time point. In the following sections, the advanc
es achieved with different types of strategies are reviewed.
Conventional strategies
Biomaterial-based bridges
Prior to the emergence of tissue engineering, strategies to build bridges for neuronal regeneration were heavily relied upon the utilization of biomaterials alone. The bridges were shaped into guidance channels with tubular structures in an attempt to protect the regenerating axons in the lumen from the external wound-healing environment, and were essentially constructed from polymeric materials, both nondegradable polymers such as silicone elastomer, polyvinyl chloride, polyethylene, expanded polytetrafluroethylene, polyvinylidenefluoride, and biodegradable polymers including polyglycolic acid, poly-lactic acid, and collagen. It is thought that tubular sleeve of a guidance channel reduces the infiltration of fibrous tissue, prov!
ides a conduit for the diffusion of neurotropic and neurotrophic factors, increases the concentration of endogenous proteins inside the channel, and presents a barrier to selectively permit or inhibit the diffusion of macromolecules between the device and the surroundings. Non-permeable and permeable guidance channels have supported regeneration and vary depending upon the materials used and the fabrication process; however, without the trophic factor support, only minimal functional recovery has been demonstrated within the mature CNS.
Fig. 2. Entubulation approach in nerve-bridging device design.
Cell- or tissue-based bridges
For quite a long period of time, cells had been avoided in the implantable constructs for tissue repair because constructs containing cellular components have problems with physical properties, management of cell phenotypes, and eliciting the host immune response. It was only recently that the advantages of cells in the reparative preclinical trials started to be recognized. Numerous studies have demonstrated the potential of cellular bridges constructed utilizing peripheral nerve grafts in promoting axonal regeneration and repair. In addition, other tissues such as blood vessels and muscle; fetal tissue grafts; different types of purified neural cells; and genetically engineered cell line to secrete trophic factors have been sho!
wn to support regeneration. However, a general drawback with the use of cellular bridges is that the environment provided by the transplanted cells may be too favorable for the regenerating axons and they fail to grow out of the bridge and reconnect with the host circuitry. Exceptions occurred with the OEGs, a highly migratory glial cell type, which was shown to have the ability to guide regenerating axons for long distances beyond the initial region of the transplant.
Tissue-engineering strategies for neuronal repair
Since many factors play roles in affecting CNS regeneration, it is unlikely that any single strategy will completely reverse the consequences of adult CNS injury. Conventional strategies that are centered on the uses of either biomaterials or cells alone have proven inadequate to elicit a significant regenerative response from severed CNS axons of the mature nervous system; as a result, approaches utilizing tissue-engineering principles have been actively pursued. A number of engineered substrates containing oriented ECM, cells, or channels have displayed potential of supporting axonal regeneration and functional recovery. Current attempts are focused on seeking new biomaterials, new cell sources, as well as novel designs of tiss!
ue-engineered neuronal bridging devices, to generate safer and more efficacious nervous tissue repairs.
Biomaterials for tissue-engineered neuronal bridging devices
Biomaterials for neuronal bridging devices include the same set of polymeric materials listed earlier. Recently, biodegradable materials adopted from surgical applications, such as polyglycolic acid (PGA), poly-l-lactic acid (PLA), and poly(lactide-co-glycolide) (PLGA) have been studied. A potential advantage offered by biodegradable materials is the disappearance from the implant site once regeneration has been completed, obviating the foreign body response and the long-term possibility of infection-related complications. Among a variety of tubular structures used for guidance channels, semipermeable hollow fiber membranes (HFMs), a widely used material for microdialysis probes and cell-encapsulation devices, appear to have a fa!
vorable implantation tissue response. However, very few studies have examined how changes in hollow fiber membrane guidance channel property and biodegradability affect tissue response and the usefulness in this regard.
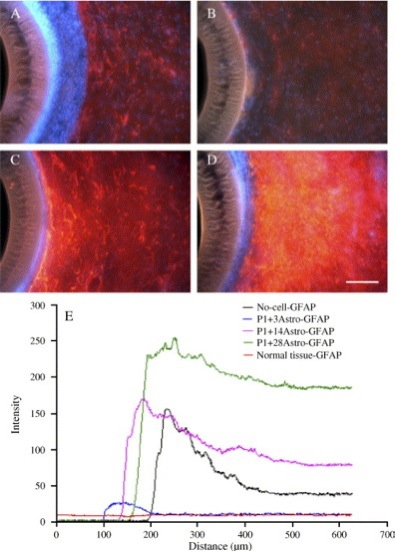
Attributing to their ability to secrete a variety of neuronotrophic factors and growth factors, such as CNTF and NGF, some types of CNS glial cells may also serve as axonal growth-permissive substrates. In particular, results from our lab have shown that transplanted astrocytes significantly intervene the adult brain tissue scar formation process in an age-dependent manner by a soluble factor-releasing mechanism. While immature astrocytes secrete soluble factors that reduce the adult brain tissue reactivity surrounding a biomaterial implant, mature astrocytes actually produce factors that enhance the gliotic response. Although the soluble factors responsible for the effect remain to be determined, these findings support the possi!
bility of using the immature astrocyte-derived scar-reductive factors within the lumen of guidance channels or near the peripheral interfaces with the host tissue to suppress the scar formation.
Fig. 3. Encapsulated astrocytes of varying ages isolated from postnatal day 1 (P1) rat and aged through different number of days in vitro (DIV) culture] ameliorate the adult rat brain tissue response to implanted hollow fiber membranes (HFMs) at 28 days post-implantation: GFAP (red)/Vimentin (green) reactivity, DAPI (blue) for nuclei. (A) Acellular control condition; (B) P1 + 3DIV astrocytes; (C) P1 + 14DIV astrocytes; (D) P1 + 28DIV astrocytes; (E) The average GFAP intensity profile of HFMs with or without co-transplanted astrocytes as a function of distance from the implant showing differences in relative abundance and organization. The membrane outer wall is at 100 μm. Scale bar = 100 μm. (For interpretation of the referenc!
es to colour in this figure legend, the reader is referred to the web version of this article.)