Nervous system injury
In the numerous attempts to integrate tissue-engineering concepts into strategies to repair nearly all of the parts of the body, neuronal repair stands out. This is partially due to the complexity of the nervous system anatomy and functioning and the inefficiency of conventional repair approaches, which were based upon single components of either biomaterials, or cells alone. In the following part, the unique anatomy of the nervous system as it pertains to axonal regeneration following injury, as well as the findings from attempts to regenerate damaged neurons and axons, are discussed.
Nervous system injury and repair
The nervous system consists of two main divisions: the central nervous system (CNS), which includes the brain and the spinal cord, and the peripheral nervous system (PNS), which is composed of cranial, spinal, and autonomic nerves that connect to the CNS. The functional unit of the nervous system is the nerve cell, also called the neuron. Each neuron has a cell body and one or more outgrowths that belong to one of two types: dendrites or axons. The dendrites serve as antenna to receive signals from the surroundings or other neurons, whereas the axons, which are usually longer than the dendrites, are engaged in transporting impulses from the cell body. Impulses can be passed from one neuron to another through the junction between !
the axon of one neuron and the dendrite of another, which is called a synapse. In addition, electrical impulse can also be directly passed from axon to axon, axon to soma, or from dendrite to dendrite.
Damage to the nervous system, caused by mechanical, thermal, chemical, or ischemic factors, can impair various nervous system functions such as memory, cognition, language, and voluntary movement. Most often, this is through crush or transection of nerve tracts. This results in the interruption of communication between nerve cell bodies and their targets. However, other types of disruption may also be important such as disruption of the interrelations between neurons and their supporting cells, and the destruction of the blood–brain barrier. Of all the types of injury, those to the CNS are among the most likely to result in death or permanent disability.
Each year, approximately 11,000 Americans suffer from spinal cord injuries and over 1.5 million people sustain some sort of brain trauma. The annual cost of medical care has been estimated at about $11 billion to treat spinal cord injuries and over $56 billion to treat brain trauma. Following injury, the PNS and the CNS respond differently. While in most cases, the severed axons of the PNS are able to re-extend and re-innervate their targets, eventually leading to functional recovery, a rare return of damaged structures and functions is observed following injuries to the CNS. As a result, disorders or injuries of the CNS are often progressive, accompanied by permanent functional impairment.
Studies have shown that the structural and functional recovery from nervous system injury depends upon a variety of factors, both intrinsic and extrinsic to the neurons. Intrinsic factors primarily involve the ability of the neurons to regenerate, whereas the extrinsic factors include the local environment at the lesion site. Comparisons between the PNS and the CNS environment following various lesions revealed that the CNS lacks several factors that are presented within the PNS. First, there are Schwann cells in the PNS, which are able to provide nutrient support, guide and myelinate regenerating axons even after axotomy of peripheral neurons, and synthesize growth-promoting molecules and growth factors. Second, a unique structu!
re in the PNS called the Bands of Bungner, containing oriented arrays of Schwann cells and their processes within a basement membrane remains after axonal and myelin degeneration, which is believed to assist the regeneration process.
Several early studies observed that retinal ganglion cells (RGCs) retained their ability to regenerate their axons when provided the fragments of peripheral nerve grafted into their damaged central pathways. Compared to PNS axons, the regenerative ability may be limited in the mature CNS in part because of extensive death that results when CNS axons become severed. Therefore, the extra permissiveness and guidance that peripheral nerves confer to the axotomized CNS axons may be the key element that determines regeneration outcome suggesting that significant differences exist in the ability of PNS vs. CNS glial to support regeneration.
The major glial cell type in the PNS is the Schwann cell, whereas in the CNS, astrocytes and oligodendrocytes are predominant. A number of studies confirmed differences in the secretory products between the PNS glial cells and CNS glial cells. Oligodendrocytes expressed proteins inhibitory to axonal outgrowth such as myelin-associated glycoprotein (MAG) and tenascin R, while Schwann cells lack these proteins. In addition, resident astrocytes in the adult CNS are activated in response to injury and participate in the scar formation process with increases in number (hyperplasia) and in size (hypertrophy). The dense scar tissue that forms following injury in the adult CNS is a formidable barrier to axonal outgrowth due, in part, to !
its dense physical structures, and the upregulation of inhibitory extracellular matrix molecules secreted by several cell types within the scar. For example, reactive astrocytes express many types of sulfated proteoglycan which inhibit axon growth, and meningeal cells also express a growth inhibitory glycoprotein, CD44, on their cell surface and invade the scar to fill in the lesion cavity.
The regenerative process in the CNS
The regenerative process in the CNS is complex, involving a cascade of sequential events. The axotomized neurons have to survive in order to regenerate, a process that can be influenced by the environment. The transected axons then have to sprout, grow into, through, and finally out of the lesion site to establish reconnections with the proper targets.
Earlier studies observed the ability of axotomized axons to sprout within 6 h of injury following the development of growth cones at the severed nerve ending. However, the sprouts only extended for up to 1 mm before growth was aborted, a phenomenon called “abortive sprouting.” The new sprouts were then gradually resorbed. The reasons for the abortive sprouting have not been fully understood, and may be explained by the inhibitory environment at the lesion site.
The next step is to transfer the axons to a permissive environment immediately following the injury. Neurotrophic factors are a group of proteins that support the survival, growth, and differentiation of neurons, and regulate synaptic plasticity both during development and in the adult CNS. An environment supplemented with neurotrophic factors may help maintain axonal sprouts and their growth toward targets. Neurotrophic factors usually upregulate in response to injury and may be responsible, at least in part, for the initial axonal sprouting. The addition of neurotrophic factors to CNS lesions can enhance regeneration..
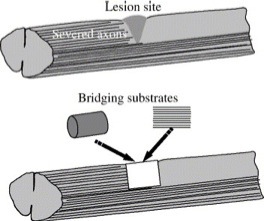
The situation becomes really challenging when it comes the time for axonal outgrowth after chronic injury. In this case, the lesion site is generally occupied by dense scar tissue filled with fibroblasts, astrocytes, meningeal cells, and other types of cells, as well as a variety of inhibitory molecules derived from them. While axons are free to be rerouted around the scar region, this may be at the expense of losing connections with their intended targets. Therefore, helping regenerating axons cross the lesion area represents an important step in the early regenerative process in which bioengineering approaches may be effective. One way to help is by creating an artificial growth-permissive substrate to connect the gap between d!
amaged nerve tracts across the scar. Such a substrate with the two ends spanning across the lesion gap is defined as a bridge or bridging substrate. A bridge can be constructed from biomaterials alone, cells or tissue alone, or a combination of biomaterials and cells based upon tissue-engineering concepts.
Fig. 1. Schematic diagram illustrating the concept of bridging following CNS injury. To restore the highly organized and aligned normal tissue architecture at the lesion site, substrates containing highly aligned architectures with scales similar to native tissue are desirable.
Normally, very few axons successfully travel through the lesion site. At this stage, patterning of guidance cues is of paramount importance. Guidance cues including both short-range cues, such as those that reside in cell membranes or extracellular matrix (ECM), and long-range cues in soluble form can be provided by molecules that communicate with the growth cones, for example, neuronal adhesion molecules, and the molecules that are known to specifically attract or repel axons, such as netrins, semaphorins, and ephrins. By exerting either chemoattraction or chemorepulsion on the growth cones, the guidance cues help confine the regenerating axons to a particular pathway. While short-range cues offer precise guidance to outgrowin!
g axons, long-range cues provide less precise guidance. The growth cones of the regenerating axons serve as both sensory structures and motor structures. They transduce attractive and repulsive cues into signals that modulate cytoskeletal dynamics and therefore determine the rate and direction of axon outgrowth and later the synapse formation by coupling the sensory and motor capabilities. Finally, when the regenerating axons reach their correct targets, stop signals are received from the guidance molecules to stop elongation and form synapses. The synaptic connections have to rearrange into a topography similar to the preinjury condition for the functional recovery to occur. It is estimated that 10% regenerated axons compared to the normal is sufficient to elicit functional recovery in rodents, reflecting substantial plasticity of the adult CNS.