Tissue Engineering: Building Bridges
Tissue engineering, as an emerging and rapidly growing field, has received extensive attention. The ultimate goal of tissue engineering as a treatment concept is to replace or restore the anatomic structure and function of damaged, injured, or missing tissue or organs following any injury or pathological process by combining biomaterials, cells or tissue, biologically active molecules, and/or stimulating mechanical forces of the tissue microenvironment. Biomaterials are fashioned into three-dimensional scaffolds to provide mechanical support, and guide cell growth into new tissues or organs. The scaffolds have to be highly porous to allow seeding of cells at high densities, and upon implantation into the body, to facilitate the i!
nfiltration and formation of large numbers of blood vessels for nutrient supply of the transplanted cells and the removal of waste products. The extracellular matrix deposited by the cells confers the physical, mechanical, and functional properties of the tissue or organ. Signals originating from the underlying substrate and the surrounding environment govern the response of the cells and their assembly into desired structures.
Biomaterial Scaffolds for Tissue Engineering
A biomaterial scaffold creates a milieu within which cells are instructed to form a tissue or organ in a highly controlled way. The principal function of a scaffold is to direct cell behaviors, such as migration, proliferation, differentiation, maintenance of phenotype, and apoptosis, by facilitating sensing and responding to the environment via cell–matrix communications and cell–cell communications. Therefore, the desirable physical characteristics of biomaterial scaffolds for tissue-engineering applications include high porosity, large surface area, large pore size, and uniformly distributed interconnected porous structures throughout the matrix. In addition, the scaffold has to provide spatial signals to modulate the orga!
nization of the cells as well as that of the extracellular matrix derived from them.
Cells for Tissue Engineering
Cells harvested from a variety of tissue sources can be used for tissue-engineering purposes. Examples include autologous cells, from the same individual; or allogeneic cells, from a different individual, but the same species; or xenogeneic cells, from a different species. Except for autologous cells, cells from other sources are likely to elicit an immune response from the recipient. The major issue with cells in tissue-engineering applications is that they have to be isolated, cultured properly, and expanded to achieve sufficient numbers of cells of a single phenotype to recover the lost function in the damaged areas.
Biological Active Molecules for Tissue Engineering
Biologically active molecules, such as cell adhesion molecules (fibronectin, laminin, collagen IV, and many others) and soluble factors (vascular endothelial growth factor, nerve growth factor, transforming growth factor, and so on), furnish the biomaterial surfaces with adhesion properties that are permissive for the attachment of particular cell types. In contrast, other cell-derived matrix molecules, such as the family of sulfated proteoglycans, are found to be inhibitory for cell growth, i.e., axonal outgrowth. Biologically active molecules interact with cells via cell surface receptors, or initiating intracellular cell signaling pathways, which trigger the expression or repression of genes, and alter the protein products tha!
t regulate cell behaviors. Selective incorporation of such biologically active molecules into the tissue-engineering constructs makes the fine-tuning of cell behaviors possible.
Environmental Cues
The mechanical forces from the surroundings represent an important aspect of the environmental cues during development of a tissue or organ. Mechanical forces, such as gravity, shear stress, compression, tension, vibration, and pressure, influence tissue development by directing cell behaviors, especially for load-bearing tissues, such as bone and cartilage. Understanding the effect of mechanical forces involved in the development and remodeling of the native tissues, and integrating this information into engineered tissues will enhance tissue replacement in vivo. The design of a biomimetic construct is highly dependent upon the structure, composition, and functions of the tissue or organ to be replaced. Many design solutions hav!
e been aimed at the delivery of signals that mimic the natural environment of the tissue or organ to be replaced via varying scaffold architecture and optimizing the spatial organization of adhesion, growth, and differentiation cues.
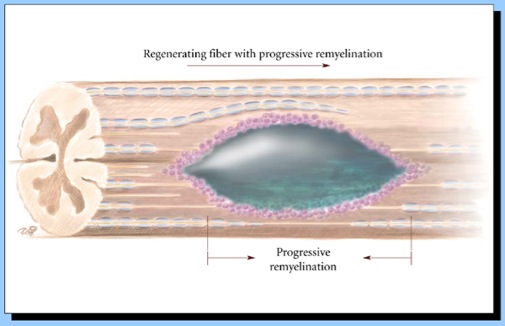
Axon Guidance for Spinal Therapy