Home General Diagnostics Information
Clinical Laboratory Diagnostics
Current Point-of-Care
Diagnostics Current
Diagnostics Companies Future Point-of-Care
Diagnostics References
Clinical Laboratory Diagnostics

(Source: http://www.cola.org)
Clinical Laboratory settings are large spaces where
medical diagnostics equipment and reagents are stored and used by clinicians to
test blood, urine, and other bodily fluids for biological abnormalities. Although
most laboratories try to carry the proper equipment and tools used to carry out
routine tests, some tests need to be outsourced to other laboratories due to
lack of personnel or testing equipment. Several clinicians work in the lab
together and need to be constantly involved in the processing and handling of
all reagents, additives, and processes. As a result, tests performed in a
laboratory can be expensive due to large facilities, expensive equipment,
clinician wages, and mass quantities of disposables and reagents used. Additionally,
many results can be time consuming to perform on the order of hours to weeks
and human errors are likely to occur.
Laboratory flow chart
Most laboratory diagnostics tests are requested by a
doctor or nurse and the patient then has to have several vials of blood drawn
which is then transported from the site of drug sampling to the central
laboratory. Labels are placed on the blood vials indicating whose blood it is
and which test is to be performed. The requests and queries of which labs to be
performed are maintained on a central computer for both the lab clinicians and
the doctors to track the process. Following some time of storage and wait, the
blood is analyzed by a laboratory clinician and lab results are given and
validated. This information is then re-entered into the central computer and
the doctor who made the request can then further analyze the results and decide
what steps to take with the patient next.
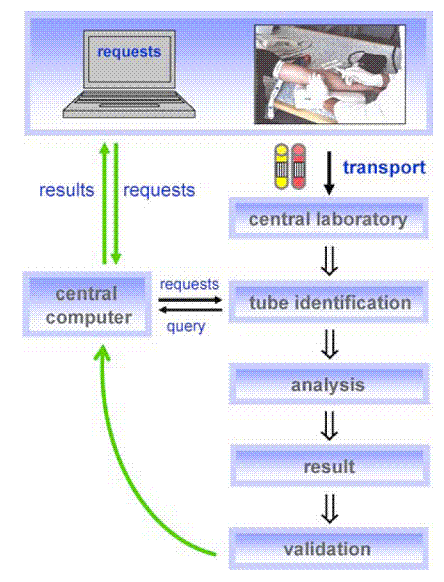
(Source: Schleicher, 2006)
Current Limitations
According to a study performed by Plebani and Carraro
in 1997, an emergency laboratory had errors in about 0.5% of the lab tests
performed. Further analysis of the laboratory process revealed that over two
thirds of these results occurred in the pre-analytical phase of the testing
phase. Meaning that most errors occurred from the time the blood was taken to
the patient, labeled, transported to the central lab, logged into the central
computer and waited to be analyzed. However, only a small percentage of the
error resulted from the actual analytical process and about 20% occurred in the
post analytical stages of validation and returning the information to the
doctor. As observed from this study, there is room for improvement in the
laboratory testing process. Not only are errors of primary concern, but often
the test results can be rendered useless if it takes too long for the
processing and analysis to occur in order for the doctor to make a timely
decision about how to treat the patient.
![]()
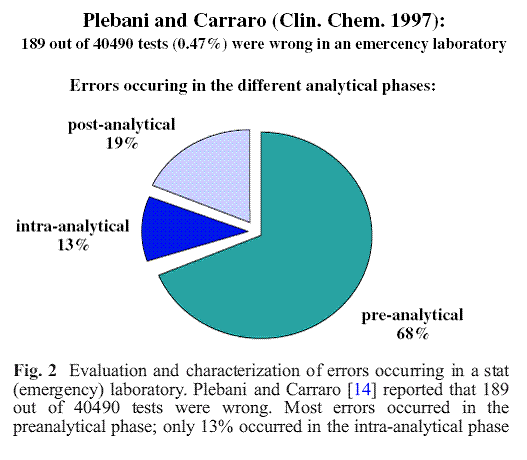
(Source: Schleicher, 2006)

(Source: onthefencefilms.com/
Home General Diagnostics Information
Clinical Laboratory Diagnostics
Current Point-of-Care
Diagnostics Current
Diagnostics Companies Future Point-of-Care
Diagnostics References