SMALL INTESTINE ENGINEERING
Using Epithelial Stem Cells
| Future Development |
![]()
This Site is Designed by Dreamweaver.
For Any Questions Contact Mahya Farnia
Different groups have presented different model for tissue engineering of the small intestine. Basic Concept: The figure below presents the schematics of the small intestine tissue engineering.
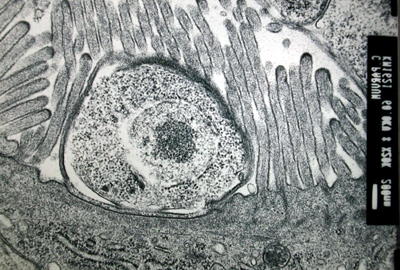
Organoid Units Implanted Subcutaneous: This novel model is to implant neonatal mouse organoid units subcutaneously and show that small intestinal-like structures would be developed. The experiments that has been done shows that after 14 days small intestine structure is formed. It was composed of a The figure below confirms that subcutaneously implanted organoid units show a temporal sequence of neomucosal morphogenesis and differentiation. The implanted organoid units induced a regeneration response with separation of cell populations at different stages of histogenesis and cytodifferentiation, shown below where (a) are goblet cells, (b) endocrine cells, (c) paneth cells, (d) entrocytes.
The success of this method in generating ectopic intestinal neomucosa illustrates that the preservation of cell interactions in the organoids is beneficial to the regeneration of mucosa from the crypt stem cells. These experiments show that postnatal organoid units, which contain small intestinal epithelial stem cells, has the capacity for small intestine regeneration when transplanted in adult recipients. Organoid Units on an Artificial matrix: In this model the transplantation of intestinal cells is developed using synthetic biodegradable polymer scaffolds to generate new intestinal tissue. This technique involves harvesting single cells and clusters of fetal organoid units on biodegradable scaffolds that would survive, proliferate, and regenerate small cystic structures lined Organoid units seeded onto polymer scaffolds can survive, vascularize, proliferate, and form cystic like structures after implantation and be surrounded by smooth muscle. In these studies,brush border enzymes are present. Cysts were anastomosed onto the native intestine after three weeks as shown below. Histological examination demonstrated that 10 weeks after anastomosis, cysts are Therefore, these two models prove that using epithelial stem cells in the organoid units, it is possible to initiate cell differentiation, and turn into small intestine. |
![]()

