



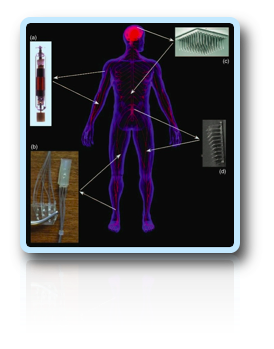
This new technology eliminates many of the problems encountered with surface stimulation. However, all implanted medical devices are foreign bodies that trigger tissue changes whose effect on device performance and the health of the recipient must be understood before they can be used clinically [2].


The acute inflammatory response usually resolves after about a week. Over subsequent months, the implant becomes encapsulated by a progressively more fibrous layer of connective tissue. If the device is highly biocompatible, the capsule is generally quite thin, and the interface between it and the device contains only a few macropha
 ges. When devices provoke continued tissue reaction, the surrounding connective tissues are thicker, more vascularized, and disorganized, and they contain large numbers of necrotic cells and leukocytes. The continued presence of leukocytes indicates possible infection and/or biological degradation of the implant itself [2].
ges. When devices provoke continued tissue reaction, the surrounding connective tissues are thicker, more vascularized, and disorganized, and they contain large numbers of necrotic cells and leukocytes. The continued presence of leukocytes indicates possible infection and/or biological degradation of the implant itself [2].



Active and passive devices elicit a similar reaction. Two lines of evidence led to the conclusion that this reaction was stable over time. First, weekly threshold measurements for eliciting muscle contractions do not change significantly over time for any of the active devices. Second, histological assessments of host tissue reveal the presence of a stable foreign-body response similar to that produced by any nontoxic biomaterial. It was also found that the host muscle used for implantation appears to affect the amount of damage caused by the implant. This observation is important because it suggests that the specific reaction of a muscle to a relatively benign implant can depend as importantly on muscle-specific characteristics as device specific characteristics. In the clinical environment, muscle stimulators will be implanted in muscles with a high degree of variation in their architectures, usage patterns, age, and health. All of these variable factors add a significant level of complexity when designing and testing a generic system that will be used for several applications [2].




Go to BME 240 Homepage


Back
Next