|
Single-Cell Isolation Array For Drug Screening and Drug Regimen Design |
| Principles of Single-Cell Analysis |
| Device Fabrication and Design |
| Uses in Clinical and Lab Settings |
| Further Reading |
| References |
| BME240 Home |
|
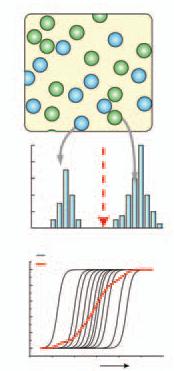
Principles of Single-Cell Analysis Drug discovery and implementation depend heavily on how cells respond to a certain stimulus. Unfortunately, cell behavior differs from manmade input-output systems in that most cellular signaling pathways are dominated by stochastic processes. The obvious solution, then, is to analyze them on a cell-by-cell basis, generating a probability distribution of possible responses to a certain perturbation. However, we do not have any ideal means of simultaneously analyzing spatial- and temoral-dependent phenomenon, especially if we are aiming for speed and efficiency. Results are further complicated by varied environments: it proved exceedingly difficult to maintain a steady environment for every single cell during an experiment, thus introducing a major confounding variable. This complication was particularly severe in devices that attempted to replicate dynamic environments. Fortunately, the microfluidics community is developing means of overcoming all of the above challenges. Below is a brief summary of established techniques for single-cell assaying, as well as an introduction to the role that microfluidics might play in the near future. Traditional techniques for cell studies
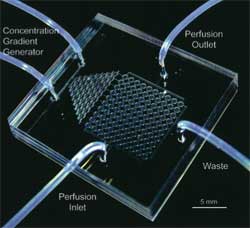
Non-Traditional Methods: The Near-Future Borrowing a page from the world of 96-well microtiter plates and cDNA arrays, engineers began to develop high-throughput means of running single-cell assays while overcoming the challenges posed by existing methods. The surge in microfluidic technology due to names such as Whitesides and Quake has meant that over the last few years, fabrication and use of such devices have become simple enough that we can envision the day when researchers can fabricate them on-site and clinicians can purchase them cheaply. Such devices come in varying degrees of complexity, but whatever the intricacy, the general advantages remain constant.
|