|
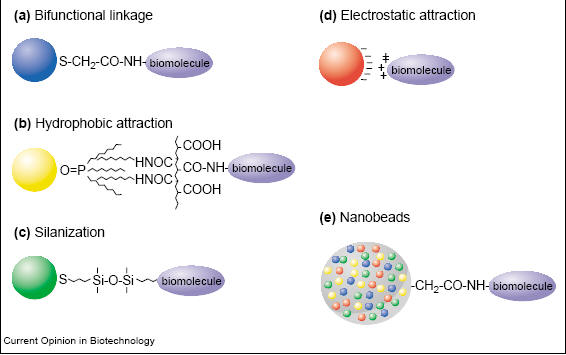
Quantum dots can be used to observe multiple systems in vivo noninvasively due to the rich surface chemistry of these dots which allows conjugation with multiple molecules. Conjugation or surface modification of quantum dots allows the tailoring of quantum dots to target desirable structures by adding peptides or markers for a specific target. The surface area of quantum dots are large enough for linking many molecules [3]. On a 4nm diameter quantum dot, it is possible to add 2 to 5 protein molecules or 50+ small molecules [3]. Biconjugation of quantum dots can be achieved through many methods such as covalent bond formation, passive adsorption, electrostatic forces, and multivalent chelation (Fig. 1) [3,6].
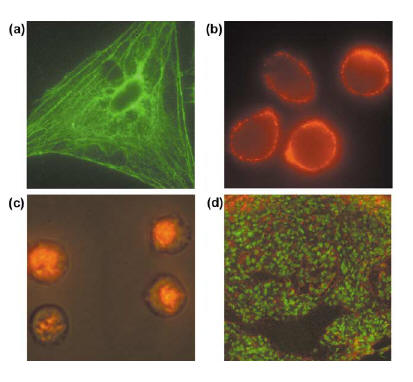
Fig. 1 Various biconjugation methods for quantum dots. a. Bifunctional linkage using mercaptoacetic acid to link quantum dots with biomolecules. b. Hydrophobic forces used to link modified acrylic acid polymer to TOPO capped quantum dots. c. Biconjugation using mercaptosilane compound. d. Electrostatic forces link negatively charged quantum dots and positively charged biomolecules. d. Quantum dots in microbeads and nanobeads [3]. The most popular cross linking reactions are carbodiimide mediated amide formation and active ester maleimide-mediated amine and sulfhydryl coupling [6]. The former method has the advantage of not requiring surface modification since proteins contain amine and carboxylic groups [6]. The latter, however, is more difficult to use since sulfhydryl groups are rare in biomolecules and are unstable in the presense of oxygen [6]. The surface of quantum dots are not spherical and contains many edges [3]. Also due to the molecules absorbed onto the surface, quantum dots are negatively charged [3]. For in vivo imaging, quantum dots would need to be water soluble and two methods have been developed to overcome this shortcoming. One method entails a silica/siloxane surface coating for ZnS-capped CdSe quantum dots [3]. This is achieved by the absorption of 3-(mercaptopropyl) trimethooxysilane onto the surface which displaces TOPO molecules [3]. The silica/siloxane shell then forms from the hydrolysis of silanol groups due to the introduction of a base [3]. The quantum dots are now soluble in intermediate polar solvents such as methanol, but reaction with a bifunctional methoxy group renders quantum dots soluble in an aqueous buffer [3]. The other method involves the adsorption of bifunctional ligands to the surface of quantum dots [3]. All of these modifications to the surface tailors the quantum dots to whatever system a person is interested in imaging. 1. Cellular imaging and tracking (Fig. 2) [6] Cellular imaging using quantum dots in vivo is important in visualizing cell migration and differentiation in real time [6]. Processes such as embryogenesis, stem-cell therapeutics, cancer metastasis, and lymphocyte immunology can be studied and tracked using quantum dots [6]. Quantum dots are taken in by cells through three processes: non-specific pinocytosis, microinjection, and peptide induced transport [2,6]. The peptide induced transport includes the use of protein induction domain of HIV-1 Tat peptide (Fig. 2c) [6]. Free quantum dots or dots which are not conjugated with a protein or peptide accumulate within vesicles in the perinuclear region after they enter the cell [2, 9]. About two billion quantum dots can be injected into the nucleus of a cell without adversely affecting the function, migration, or differentiation of the cell [2,6]. Studies have shown that single cellular structures can be visualized and tracked which would not be possible with organic dyes [6]. The sensitivity of single molecules achieved through quantum dots are useful for studying receptor diffusion dynamics, signaling pathways, enzyme activity, molecular motors, biomolecular transport, and ligand-receptor interactions [6]. Quantum dots have also shown great success in immunofluorescence labeling of live cells [9].
Fig. 2 Fluorescence staining of cells and tissue. (a). Actin staining of 3T3 fibroblast cells. (b). QD -antibody conjugate targeting live MDA-MB-231 breast tumor cells. (c). QD-Tat peptide conjugate targeting intracellular live mammalian cells. (d). QD staining of frozen tissue specimen. [6] The first study using quantum dots for live cell imaging was done by Nie et al using transferrin protein conjugated dots taken up by Hela cells through the receptor mediated mechanism [9]. Dubertret et. al. placed quantum dots into Xenopus embryos through microinjection to track the stages of embryogenesis [2, 9]. Also studies on motility and migration of cancer cells were conducted using quantum dots [2]. Rosenthal et. al. studied the serotonin transport mechanism through the use of quantum dots [2,10]. Quantum dots linked to serotonin were able to recognize and label serotonin neurotransmitters in cell membranes as well as inhibit serotonin transportation which mimicked closely the actions of free serotonin, in effect proving to be a valuable probe [2,10]. Other pathways studied using quantum dots include glycine receptors and erb/HER receptor mediated signal transduction [2,9]. Dahan et al were able to track glycine receptors for 20min while with Cy3 the duration was only 5s [2,9,10]. Quantum dots were also successful in determining the binding and internalization kinetics of EGF-QD which are used to observe transmembrane receptor tyrosine kinases dependent signaling [9]. Quantum dots have also proven helpful in initiating specific physiological and pharmacological events in cells such as cell apoptosis [9].
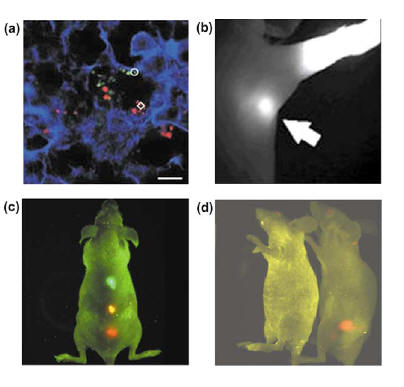
2. Tissue imaging (Fig. 3) Several areas of tissue imaging has shown success with quantum dots. One area is lymph node mapping and vascular imaging such as blood pool imaging in vivo [6]. Webb et al used PEG-coated quantum dots to visualize small blood vessels [6]. PEG-coated quantum dots stayed within circulation longer (half life about 3 hrs) than organic dyes which are eliminated from circulation within minutes of injection [6]. The PEG coating makes the quantum dots large enough to avoid renal filtration, but are small enough and hydrophilic enough to avoid opsonization and reticuloendothelial uptake [6,10]. Kim et al were successful in clearly imaging and delineating sentinel lymph nodes during surgery within rat models (Fig. 3b) [6,10]. Kim et al utilized the properties of near infrared imaging or NIR since NIR minimizes autofluorescence of endogenous chromophores because chromophores within living tissues absorb light more effectively from the visible light regime and less from the infrared region [10]. Also NIR imaging penetrates deeper into tissues than visible light which would be desirable for imaging blood vessels and lymph nodes within the body [10]. NIR imaging using quantum dots was achieved by utilizing novel core-shell nanostructures called type II quantum dots [6].
Fig. 3(a). QD labelled cancer cells stained ex vivo trapped inside of mouse lung tissue. (b). In vivo labeling of sentinel lymph nodes. (c) In vivo imaging of multi-color QD microbeads injected into a live mouse. (d). In vivo imaging of prostate cancer in mouse using QD probes. [6] Tumors can be targeted by quantum dots through a passive and active mechanism [6]. The passive mechanism is possible through the preferential accumulation of quantum dots in tumor sites due to enhanced permeability and retention effect [6]. Two factors are believed to cause this phenomenon. One factor is the increased release of vascular endothelial growth factors or VEGF which causes the vasculature of the tumor to become leaky or very permeable [6]. This results in the leakage of small particles and macromolecules into the tumor from the blood vessels [6]. The other factor which causes the preferential accumulation is the lack of an effective lymph drainage system in a tumor which causes accumulation of quantum dots in the tumor [6]. The active mechanism entails conjugating the quantum dots with antibodies or other molecules specific for tumors [6]. Quantum dots were conjugated with an antibody specific for prostate cancer PMSA (prostate-specific membrane antigen) and the tumor was visualized by Gao et al (Fig. 3d) [6,10]. PMSA is currently used for diagnostic and therapeutic purposes for prostate cancer and the accumulation of PMSA at the site of tumor is used to treat prostate cancer [6]. Gao et al were also successful in creating a multi-functional quantum dots for imaging and targeting of tumor cells [6]. The quantum dot contains ligands that target tumors, PEG to increase circulation time and biocompatibility, and an amphiphilic triblock copolymer to prevent particle aggregation and fluorescence loss in vivo [6].
|