|
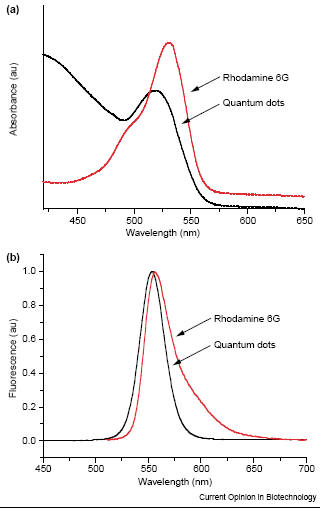
fluorophores Quantum dots offer several advantage over organic fluorophores which are commonly used for in vivo labeling. Quantum dots have the ability to have a wide range of optical properties not present in organic fluorophores such as rhodamine 6G and fluorescein. Organic fluorphores have a narrow absorption spectrum which results in a narrow range of emission [2,3]. Also the organic dyes do not have a sharp symmetric emission peak which is further broadened by a red-tail [2,3]. In contrast, quantum dots have a broader excitation spectra and a narrow more sharply defined emission peak [2]. Due to these properties, a single light source can be used to excite multicolor quantum dots simultaneously without signal overlap (Fig.1) [2,3].
Fig. 1 The absorption (a) and emission (b) spectra of an organic dye (rhodamine 6G, red line) and quantum dots (black line). The organic dye has a narrower absorption and narrower fluorescence spectra while for quantum dots it is vice versa [3]. Quantum dots also have a brighter emission and a higher signal to noise ratio compared with organic dyes [1]. The brightness of quantum dots compared to organic dyes are 10-20 times brighter [6]. Quantum dots are stable flurophores due to its inorganic composition which reduces the effect of photobleaching compared to organic dyes (Fig.2) [2,3]. The fluorescence time for quantum dots is about 10 to 40 ns which is longer than the fluorescence of a few nanoseconds of organic dyes (Fig. 3) [2,3,4]. The inorganic composition of quantum dots also make them more robust toward metabolic degradation which contributes to their longevity in vivo [10]. The large Stokes shift (difference between peak absorption and peak emission wavelengths) reduces autofluorescence which increases sensitivity [10]. In vivo imaging of thick specimens also benefits from the stability and brightness of quantum dots because high absorption, light scattering, and autofluorescence from endogenous chromophores can obscure the signal [7]. Quantum dots have allowed in vivo imaging of tissue of large animals such as sentinel lymph nodes of pigs (1 cm deep) and their self illuminating property which require no external light source have allowed background free imaging [7]. Lastly, the multicolor property of quantum dots allows the use of many probes to track several targets in vivo simultaneously [6] and the yield obtained from quantum dots is 89% at room temperature [10].
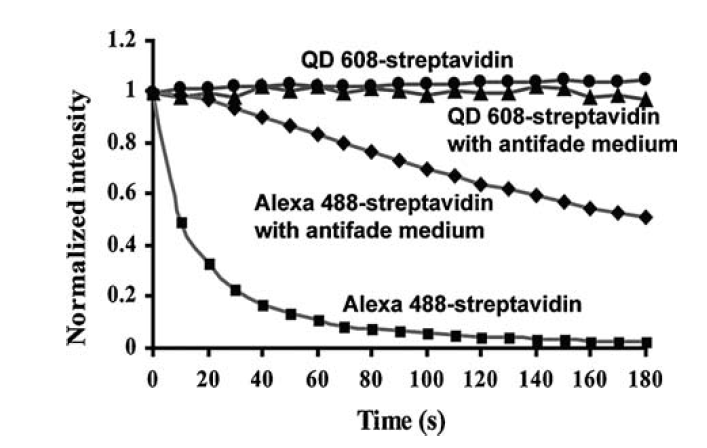
Fig. 2 Comparison of fluorescence intensity of organic dyes and quantum dots. The fluorescence intensity of quantum dots do not diminish with time while organic fluorophores lose their intensities in 20s [2]. Photobleaching is reduced or nonexistent for the quantum dots while the organic dyes show considerable photobleaching [6].
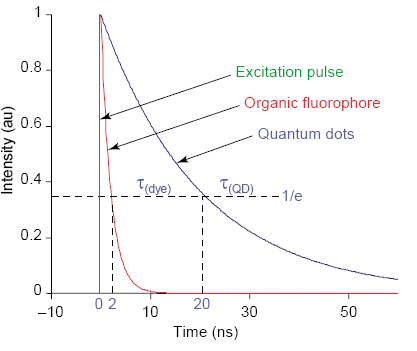
Fig. 3 The excitation decay of organic fluorophores and quantum dots. The time constant of the quantum dot is longer than the time constant of the organic fluorophore. [6]
|