Non Small-Cell Lung Cancer
Non small-cell lung cancer is responsible for 80-85% of clinical lung cancer cases. The traditional treatment options for this type of cancer include chemotherapy or surgery, however, surgery is generally only used in earlier stages of the disease, and chemotherapy often has many undesirable side effects. Genetic testing is enabling the detection of specific mutations in patients, which guide physicians to a more effective treatment option. Several different mutations in the epidermal growth factor receptor (EGF-R) are found in NSCLC patients. Researchers are beginning to treat patients by suggesting a particular drug, based on the patient's particular mutations, often leading to longer survival and much milder side effects than with more traditional treatment options. These categories of drugs include monoclonal antibodies and small molecule inhibitors of receptors. A few examples are described below.
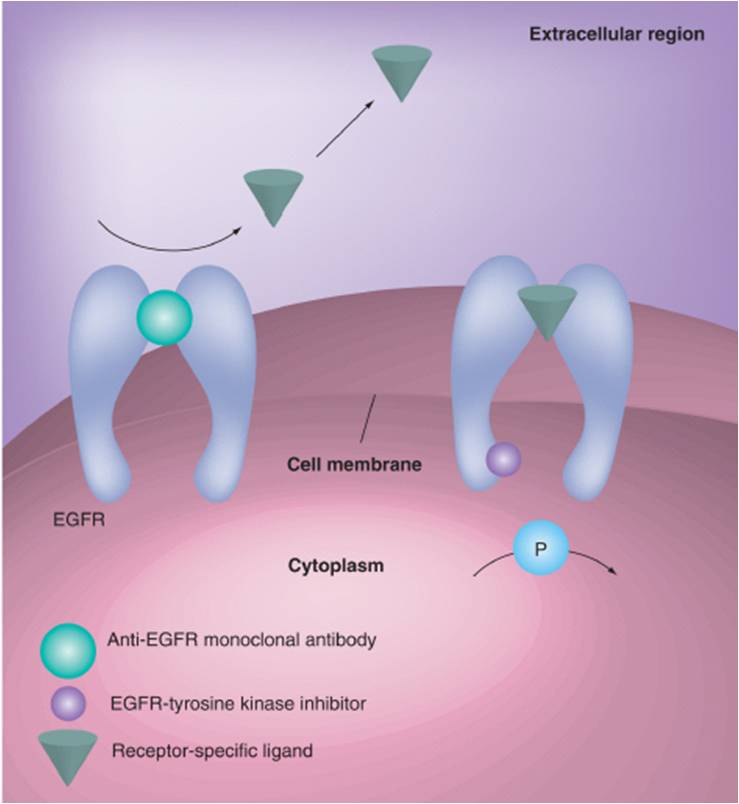
Table of Contents
Monoclonal Antibodies
This type of treatment prevents signal transduction along the epidermal growth factor receptor (EGFR) pathway, which leads to lung cancer. Monoclonal antibodies are a purified set of polyclonal antibodies, which bind to only a single epitope on the target molecule. Ensuring that the antibodies only bind to a single location on the receptor prevents binding of epidermal growth factor to its receptor, preventing the deleterious downstream signaling effects of this event. In addition to blocking signaling pathways that would lead to cancer progression, monoclonal antibody binding to these receptors recruits macrophages and monocytes in the periphery, which can eventually destroy the cancer cell.
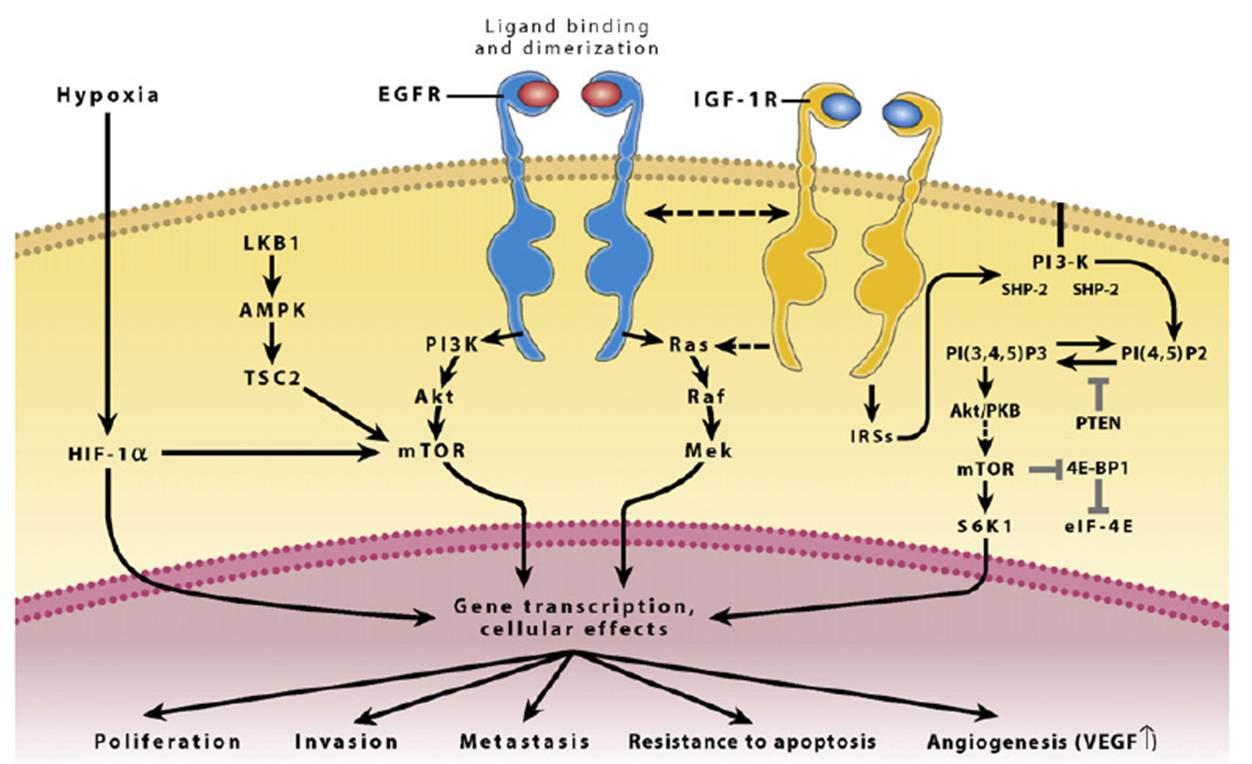
The image above shows signaling pathways for EGFR (epidermal growth factor receptor), the misregulation of which is implicated in many cases of NSCLC. (Image taken from Dempke et al.)
For treatment with a monoclonal antibody against EGFR (Cetuximab), patients are screened for the presence of mutations on the tyrosine kinase domain of the EGF receptor using immunohistochemistry (proteomics). While this drug has not yet been FDA approved for treatment of NSCLC, recent studies have shown its efficacy in prolonging patient survival, when used in conjunction with, or compared to chemotherapy.
[back to top]
Small Molecule/Tyrosine Kinase Inhibitors of Receptors
For patients who are shown to have certain types of mutations in the EGF receptor, the receptor becomes more sensitive to inhibitors. The mutation is either a deletion in exon 19 or a single missense mutation (of arginine to leucine) at the 858th codon. The small molecule drugs gefitinib and erlotinib have shown much promise in treating patients with these mutations. In the case of these drugs, certain mutations in the EGF receptor (such as the T790M mutation) will render these drugs ineffective against the cancer. This underscores the importance of genetic testing both for identifying potentially effective and ineffective treatment options.
Small molecule inhibitors of a receptor known as c-MET may also provide treatment for NSCLC. This receptor, which binds hepatocyte growth factor, has been shown to contribute to tumorigenesis when incorrectly regulated. A majority of NSCLC patients (60-80%) overexpress c-MET, and 20% of NSCLC patients have a mutation in this receptor. Ten compounds are currently under commercial development as c-MET inhibitor molecules. The combination of delivering EGF-R and c-MET receptor inhibitor drugs has also been shown to improve patients outcomes over the administration of one of these drugs alone.
[back to top]
EGF-R Copy Number
Information about the EGF-R copy number can be obtained by fluorescent in-situ hybridization (FISH), immunohistochemistry, or measured directly by DNA sequencing. Using this information, physicians can predict how well a patient may respond to a certain drug. A high copy number of EGF-R, determined from DNA sequencing, predicts greater response to treatment with gefitinib, in those patients than patients with a lower copy number. Similarly, patients taking the monoclonal antibody drug cetuximab, response to the drug was higher in a group of patients who were positive in a FISH test for EGF-R.
[back to top]