Bubble Ultrasound-Guided Biopsy/ Surgery
BME 240
| |
| Home |
| Background |
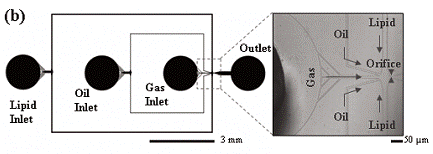
| Applications |
| Future |
| References |
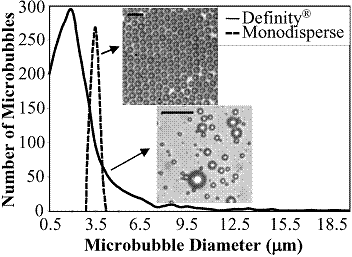
| Background |
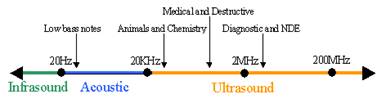
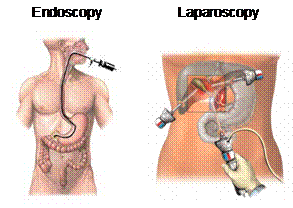
Ultrasound The medical imaging technique, sonography, is the most widely known application of ultrasound. Sonography is used to image the size and structure of muscle, tendons, and internal organs in addition to fetuses and pathological lesions. In comparison to other medical imaging technologies such as CT and MRI, ultrasound is not only much less expensive, but mobile. Although ultrasonic energy produced by ultrasound has two possible physiological effects including heating soft tissue and causing minute inflammation, it is considered to be a very safe test. Continual use over long durations is for either CT or MRI is impractical, but plausible for ultrasound. Sonography, in particular, maintains a continuous picture of a monitor using high frequency sound waves between 3.5 and 7 megahertz. The signal is emitted from a transducer probe that is placed in contact with the specimen surface. When ultrasound meets an interface of differing echogenicity, the sound wave is either reflected, refracted or absorbed. The transducer, though emitting ultrasound in rapid pulses, also acts as a receiver for reflected sound waves, which are processed into an image. The thickness, size and location of various soft tissue structures in relation to the origin of the ultrasound beam are calculated at any point in time. The strength of the reflected sound wave depends on the difference in acoustic impedance which relates to tissue density. The greater the difference in acoustic impedance between two adjacent tissues the more reflective will be their boundary. Higher frequency ultrasound waves have a longer near field and less divergence in the far field; they permit better resolution of small structures. More energy however is absorbed and scattered by the soft tissues so that higher frequencies have less penetrating ability. Conversely, a transducer producing lower frequencies will provide greater depth of penetration but less well defined images. Focusing and aperture control technology are often employed to narrow the beam along its entire path to achieve maximum lateral resolution. [2] Endoscope Endoscopy is used to view the interior of the body through natural opening or in cavities accessed by minimal surgical incisions. The most common application of endoscopes includes diagnostic examination and laparoscopic surgery. Fiber-optic endoscopes have shafts composed of hair-like glass rods bundled together, which allow light to travel from one end to the other by means of internal reflection. The light allows the camera to view what is in front of the endoscope. The shaft is pliable, enabling the user to maneuver into the canals of the body, such as the gastrointestinal tract. Accessories can be added to the end of the endoscope, making it possible to collect tissue/cell specimens or perform surgical procedures, such as resection of ablation. It is this possibility that greatly increases the potential application of endoscopic guided surgery. [5] Microbubbles Current microbubbles used in surgical procedures are generated in mass by machines that shake albumin or saline solution with air. One recent improvement was the mass production of microspheres with lipid using the commercially available product, Definity by Lantheus Medical Imaging, Inc. However, with all the current options the ideal microbubble is still not available. Research and development of new method for producing microbubbles utilizes microfluidic platforms. Such devices produce micorbubbles one at a time with high homogeneity and tailored specifications. [8] Important features of the microbubbles include size and targeting capabilities. Since microbubbles made in microfluidic device are produced one at a time from the same orifice, the size distribution is extremely monodisperse. The figure below compares microbubbles from one such microfluidic device to microbubbles from the Definity, revealing an obvious difference in monodispersity. The more monodisperse the microbubble sample the more effective the ultrasound imaging, since a single sound wave is used to interact with the tissue and bubbles. [10] Microbubbles can be made to target specific tissue by outfitting them with ligands that bind specific receptors expressed by cell types of interest. These ligand-receptor pairs can be used to target cancerous cells expressing a specific receptor profile. Current microbubbles being developed for this purpose have perfluorocarbon gas core and lipid shell cover with a polyethylene glycol (PEG) layer. The PEG layer increase stability and helps prevent an immune response. [9] The latest break through in microbubble production is multilayer bubbles. This increases stability and the possibility of ligand or drug incorporation. [8] |
Figure 1. Ultrasound frequencies
Figure 3. Common use of endoscope
Figure 4. Microbubble production by microfluidic device [8]
Figure 5. Comparison of microbubble size distribution from microfluidic device and Definity [10]
Figure 6. Multilayer microbubble production from microfluidic device [8] |