Sepsis and Septicemia, Treatment and the link to Nanotechnology
Sepsis is a pathological state resulting from the presence of microorganisms or their poisonous products in the bloodstream. Microbial infection may manifest as cellulitis (local dissemination of infection), lymphangitis or lymphadenitis (dispersion along lymphatic channels) or septicemia (widespread dissemination via the bloodstream). Septicemia, also known as blood poisoning, is the presence of pathogenic microorganisms in the blood. If allowed to progress, these microorganisms may multiply and cause an overwhelming infection. Symptoms include chills and fever, petechiae (small purplish skin spots), purpuric pustules and abscesses. Acute septicemia, which includes tachycardia, tachypnea, and altered mental function, may combine with hypotension and inadequate organ perfusion as septic shock -- the resulting decreased myocardial contractility and circulatory failure can lead to widespread tissue injury and eventually multiple organ failure and death, often in as few as 1-3 days. Risk is especially high for immune-compromised individuals. Asplenic patients are particularly susceptible to rapidly progressive sepsis from encapsulated microorganisms such as streptococcal pneumonia, hemophilus influenza and meningococcus, and will die if the infection is not recognized rapidly and treated aggressively.Septicemia may be caused by several different classes of pathogenic organisms, most commonly identified as bacteria - bacteremia, viruses - viremia, fungi - fungemia, parasites - parasitemia, and rickettsiae - rickettsemia.
Bacteremia
The healthy human bloodstream is generally considered a sterile environment. Although bacterial nutrients are plentiful in blood, major antimicrobial defenses include the circulating neutrophils and monocytes capable of phagocytosis and the supporting components of humoral immunity including complement and immunoglobulins.
Still, it is not unusual to find a few bacteria in blood. Normal activities like chewing, brushing or flossing teeth causes movement of teeth in their sockets, infusing a burst of commensal oral microbes into the bloodstream]. Bacteria can enter the blood via an injury to the skin, the lining of the mouth or gums, or from gingivitis or other minor infections in the skin and elsewhere. Bacteremias from a focus of infection are usually intermittent, while those from vascular system infection tend to be continuous, such as endocarditis or embolism from heart valve vegetations in subacute bacterial endocarditis (SBE), sometimes leading to infectious mycotic (e.g., Staphylococcus aureus) aneurysms.
Bacteria can also enter the blood during surgical, dental, or other medical procedures such as the insertion of I.V. lines (providing fluids, nutrition or medications), cystoscopy (a viewing tube inserted to examine the bladder), colonoscopy (a viewing tube inserted to view the colon), or heart valve replacement with a prosthetic (thankfully now rare, due to heavy preoperative dosing with cefazolin). Such bacteria are normally removed by circulating leukocytes (along with the fixed reticuloendothelial cells in the spleen, liver, and lungs), but a few species of bacteria are unusually virulent and can overwhelm the natural defenses. The CDC estimates that ~25,000 U.S. patients die each year from bacterial sepsis. Worldwide, there are ~1.5 million cases of sepsis and ~0.5 million deaths from sepsis annually.
Bacteria are unicellular microorganisms capable of independent metabolism, growth, and replication. Their shapes are generally spherical or ovoid (cocci), cylindrical or rodlike (bacilli), and curved-rod, spiral or comma-like (spirilla). Bacilli may remain associated after cell division and form colonies configured like strings of sausages. Bacteria range in size from 0.2-2 microns in width or diameter, and from 1-10 microns in length for the nonspherical species; the largest known bacterium is Thiomargarita namibiensis, with spheroidal diameters from 100-750 microns. Many spherical bacteria are ~1 micron in diameter; an average rod or short spiral cell might be ~1 micron wide and 3-5 microns long. However, most bacteria involved in bacteremia and sepsis are <2 micron3 in volume.
Viremia
Viremia is the presence of virus particles in the bloodstream, usually a transient condition. Viruses are acellular bioactive parasites that attack virtually every form of cellular life. Viruses have diameters ranging from 16-300 nm -- for example, poliomyelitis ~18 nm, yellow fever ~25 nm, adenovirus (common cold) ~70 nm, influenza (flu) ~100 nm, herpes simplex and rabies ~125 nm, and psittacosis ~275 nm. Their shape is either pseudospherical with icosahedral symmetry, as in the poliomyelitis virus, or rodlike, as in the tobacco mosaic virus (TMV). A virus surrounded only by protein coat (capsid) is a naked virus; some viruses (e.g., HIV, HSV, pox), called enveloped viruses, acquire a lipid membrane envelope from their host cell upon release.
Fungemia
In severely immunocompromised patients, fungi may gain access to the bloodstream, producing fungemia. Fungal cells in peripheral blood are typically ovoid to elongated, from 3 × 3 microns up to 7 ×10 microns in size, and occur singly, budding, or in short chains and clusters. Candidal fungemia is most common.
Parasitemia
Parasitemia arises from parasites that have evolved to live in the bloodstream include the Plasmodium (malaria) family and the flagellate protozoans Trypanosoma (sleeping sickness) and Leishmania (leishmaniasis). Blood parasites typically have a juvenile form that is ovoid or ring-shaped with dimensions of 1-5 microns, and an adult tubular form measuring 1-5 microns in width and 10-30 microns in length.
Rickettsia
Rickettsia are rod-shaped or coccoid gram-negative obligate intracellular parasites ~0.25 microns in diameter that in humans grow principally in endothelial cells of small blood vessels, producing vasculitis, cell necrosis, vessel thrombosis, skin rashes and organ dysfunctions. The infection is characterized by repetitive cycles of bloodborne organisms, or rickettsemia.
Treatment
This disorder must be treated in a hospital, usually with admission to an intensive care unit. Intravenous (IV) fluids are given to maintain the patient's blood pressure. Strong IV drugs called sympathomimetics are often needed to maintain the blood pressure. Oxygen therapy is begun to maintain oxygen saturation. The infection is treated with broad spectrum antibiotics (those that are effective against a wide range of organisms) before the organism is identified. Once cultures have identified the specific organism that is responsible for the infection, antibiotics that are specific for that organism are begun. Plasma or other treatment may be needed for correction of clotting abnormalities. Even though treatment is available often times complications can occur including:
- Septic shock
- Impaired blood flow to vital organs (brain, heart, kidneys)
- Disseminated intravascular coagulation
- Waterhouse-Friderichsen syndrome
- adult respiratory distress syndrome (ARDS)
In addition often times the treatments require months to years of daily doses, and antibiotic resistant microbes are becomes more prevalent. Septic shock has a high death rate, exceeding 50%, depending on the type of organism involved. The organism involved and how quickly the patient is hospitalized will determine the outcome.
Link to Nanotechnology - Phage Therapy
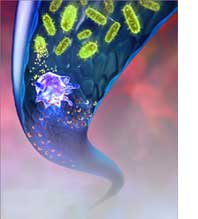
 An interesting emerging alternative to antibiotic therapy -- and a small step towards nanomedicine -- is phage therapy. Bacteriophage viruses are tiny biological nanomachines that were first employed against bacteria by d'Herelle in 1922 but were abandoned therapeutically (and then superceded by antibiotics) after disappointments in early trials. Bacteriophages may be viewed as self-replicating pharmaceutical agents that can consume and destroy pathogenic bacteria when injected into infected hosts. The picture at the right shows a phage on a microbe injecting phage proteins that become more phages to eliminate the microbes that can cause infections and diseases. A single E. coli cell injected with a single T4 phage at 37°C in rich media lyses after 25-30 minutes, releasing 100-200 phage particles; if additional T4 particles are added >4 minutes after the first, lysis inhibition is the result and the bacterium will produce virions for up to 6 hours before it finally lyses. With the relatively recent realization that phages have a very narrow host range, success rates of 80-95% have been reported and interest in phage therapy as an alternative to antibiotics is reawakening. One practical difficulty with phage therapy is that even in the absence of an immune response, intravenous therapeutic phage particles are rapidly eliminated from circulation by the reticuloendothelial system (RES), largely by sequestration in the spleen. A possible solution that is based on this idea is the use of microbivores - nanorobots that act as cleaners for the body.
An interesting emerging alternative to antibiotic therapy -- and a small step towards nanomedicine -- is phage therapy. Bacteriophage viruses are tiny biological nanomachines that were first employed against bacteria by d'Herelle in 1922 but were abandoned therapeutically (and then superceded by antibiotics) after disappointments in early trials. Bacteriophages may be viewed as self-replicating pharmaceutical agents that can consume and destroy pathogenic bacteria when injected into infected hosts. The picture at the right shows a phage on a microbe injecting phage proteins that become more phages to eliminate the microbes that can cause infections and diseases. A single E. coli cell injected with a single T4 phage at 37°C in rich media lyses after 25-30 minutes, releasing 100-200 phage particles; if additional T4 particles are added >4 minutes after the first, lysis inhibition is the result and the bacterium will produce virions for up to 6 hours before it finally lyses. With the relatively recent realization that phages have a very narrow host range, success rates of 80-95% have been reported and interest in phage therapy as an alternative to antibiotics is reawakening. One practical difficulty with phage therapy is that even in the absence of an immune response, intravenous therapeutic phage particles are rapidly eliminated from circulation by the reticuloendothelial system (RES), largely by sequestration in the spleen. A possible solution that is based on this idea is the use of microbivores - nanorobots that act as cleaners for the body.


 An interesting emerging alternative to antibiotic therapy -- and a small step towards nanomedicine -- is phage therapy. Bacteriophage viruses are tiny biological nanomachines that were first employed against bacteria by d'Herelle in 1922 but were abandoned therapeutically (and then superceded by antibiotics) after disappointments in early trials. Bacteriophages may be viewed as self-replicating pharmaceutical agents that can consume and destroy pathogenic bacteria when injected into infected hosts. The picture at the right shows a phage on a microbe injecting phage proteins that become more phages to eliminate the microbes that can cause infections and diseases. A single E. coli cell injected with a single T4 phage at 37°C in rich media lyses after 25-30 minutes, releasing 100-200 phage particles; if additional T4 particles are added >4 minutes after the first, lysis inhibition is the result and the bacterium will produce virions for up to 6 hours before it finally lyses. With the relatively recent realization that phages have a very narrow host range, success rates of 80-95% have been reported and interest in phage therapy as an alternative to antibiotics is reawakening. One practical difficulty with phage therapy is that even in the absence of an immune response, intravenous therapeutic phage particles are rapidly eliminated from circulation by the reticuloendothelial system (RES), largely by sequestration in the spleen. A possible solution that is based on this idea is the use of microbivores - nanorobots that act as cleaners for the body.
An interesting emerging alternative to antibiotic therapy -- and a small step towards nanomedicine -- is phage therapy. Bacteriophage viruses are tiny biological nanomachines that were first employed against bacteria by d'Herelle in 1922 but were abandoned therapeutically (and then superceded by antibiotics) after disappointments in early trials. Bacteriophages may be viewed as self-replicating pharmaceutical agents that can consume and destroy pathogenic bacteria when injected into infected hosts. The picture at the right shows a phage on a microbe injecting phage proteins that become more phages to eliminate the microbes that can cause infections and diseases. A single E. coli cell injected with a single T4 phage at 37°C in rich media lyses after 25-30 minutes, releasing 100-200 phage particles; if additional T4 particles are added >4 minutes after the first, lysis inhibition is the result and the bacterium will produce virions for up to 6 hours before it finally lyses. With the relatively recent realization that phages have a very narrow host range, success rates of 80-95% have been reported and interest in phage therapy as an alternative to antibiotics is reawakening. One practical difficulty with phage therapy is that even in the absence of an immune response, intravenous therapeutic phage particles are rapidly eliminated from circulation by the reticuloendothelial system (RES), largely by sequestration in the spleen. A possible solution that is based on this idea is the use of microbivores - nanorobots that act as cleaners for the body. 