Novel Method for Treating Myocardial Infarction using a Cardiac Patch
Introduction
Researches are interested in revascularizing the damaged myocardium in patients with coronary artery disease. Conventional treatments aim to prevent an additional myocardial infarction by attempting to prevent another atherosclerotic blockage and assuring continual blood flow. However, these conventional treatments to not aid in the revascularization of the damaged myocardium [8]. This approach is attempting to develop methods to promote revascularization. It has been shown that the use of safe angiogenic therapies to induce revascularization have been successful. Such factors as VEGF (vascular endothelial growth factor), GMCSF (granulocyte-macrophage colony stimulating factor), as well as bFGF (basic fibroblast growth factor) have successfully induced stimulation to micro-vessel growth and thus increased blood flow through the myocardium [8].
What researches are striving toward is combining these angiogenic therapies with a 3-dimensional tissue engineered construct to implant over a region of the myocardial infarct as a patch. This patch will attempt to stimulate the formation of a mature microvascular network [8]. This network will be made up of arterioles, capillaries and venules. Various approaches are currently being studied and this page will serve as a review of some of the current methods [8].
3DFC (3-Dimenstional Fibroblast Culture)
Dr. Robert Keller proposed the use of a scaffold based, three-dimensional, human dermal fibroblast culture as a cardiac patch in order to stimulate revascularization of the left ventricle post myocardial infarction. Specifically, the human tissue was Dermagraft (Smith&Nephew, London, UK). This Dermagraft is a human tissue composed of human dermal fibroblasts that were cultured on 5x7 cm pieces of knitted Vicryl mesh [8].
This tissue engineered construct contains structural extracellular matrix proteins and viable cells that will secrete angiogenic growth factors. Some of the secreted factors are VEGF, bGFG, and HGH, all of which have demonstrated angiogenic benefits [8].
The goal of this patch will not only to be to improve angiogenesis but to improve left ventricular function in the infarcted heart overall. Ideally this patch will slow the reduction of function of the left ventricle and over time improve its function overall [8].
Conclusions: As depicted by the chart it appears as if those infarcted hearts treated with the 3DFC patch have less ventricular wall thinning, smaller infarct area, and no aneurysm formation. As opposed to those untreated infarct hearts that have thinned myocardium with a large aneurysmal section. The data also showed that the hearts treated with the 3DFC had significantly higher Ejection Fractions, significantly higher preload recruitable stroke work. Therefore, it was concluded that the 3DFC patch attenuated further loss of the left ventricle function and this may be related to myocardial revascularization of the myocardium [8].
Human Bone Marrow-Derived Cells and Collagen Cardiac Patch
This italian group declares that transplanting hematopoietic and peripheral blood derived stem/progenitor cells can be beneficial in slowing the negative effects of heart failure. This group proposed to deliver human bone marrow CD133+ derived cells to a tissue engineered type I collagen patch placed on the injured hearts [11]. They studied whether these cells could convert both in vivo and in vitro into cardiac myocytes. Although there was no sign on in vivo cardiac myocyte differentials, there were signs of new formation of capillaries and small arterioles. As a control they also injected cells alone into the myocardium [11].
This group concluded:
-
1)In vitro the the BM-CD133+ cells displayed limited propensity to convert to cardiac myocytes.
-
2)The vascularized scaffold did not cause selective differentiation or an advantage for the previously described conversion to cardiac myocytes.
-
3)Although patching and cell transplantation is beneficial for angiogenesis and arteriogenesis, it did not produce better results for endothelial and smooth muscle cell differentiation when compared to traditional injection directly into the myocardium [11].
Vascularized Cardiac Patch:
Methods of In Vivo Pre-Vasculatization:
Various groups have utilized different methods to prevascularize a tissue engineered scaffold. One group in particular in Israel headed by Dr.Tal Dvir, created a cardiac patch by seeding neonatal cardiac cells with Matrigel and angiogenic factors into an alginate scaffold that had sustained release capabilities [4,5]. This scaffold was then cultured for 48h in vitro. Next, to induce patch vascularization the patch was implanted into a rat omentum for 7 days. The omentum is a large fold of the peritoneum that hangs down from the stomach and extends to the abdominal wall. The thought was that the omentum is very rich in blood vessels and therefore would provide a good place to induce the growth of mature vasculature [4,5].
After being vascularized on the omentum, the patch was transplanted onto infarcted rat hearts. After 28 days results were collected. It was found that the vascularized patch: 1) Showed structural integration and electrical integration into the host myocardium 2) Vascularized patch induced thicker scars 3) Prevented further dilation of the chamber. Therefore their study concluded that prevascularizing the cardiac patch can overall improve cardiac function following a myocardial infarction [2,4,5].
Methods utilizing In Vivo Pre-Vascularization:
The work by Lesman et al (2009) attempted to reestablish the structure and function of the injured myocardium by cardiac tissue engineering. Dr. Lesman combined cardiac myocytes derived from human embryonic stem cells, HUVECs (Human umbilical vein endothelial cells), and MEFs (Mouse embryonic fibroblasts) into the three-dimensional biodegradable scaffold in attempt to recreate the early embryonic cardiac development [2,5]. Additionally, an important component of this work is that this tissue engineered vascularized cardiac muscle although generated ex vivo can function in vivo. The group found that prevascularizing the graft can be extremely beneficial to avoid hostile ischemic environments where oxygen and nutrients cannot diffuse throughout the entire scaffold [5]. Therefore vascularization is crucial to this success. Especially in the case of the heart where there is a high metabolic demand [5].
Additionally, it was found that the use of the triculture was substantially more effective than using just cardiac myocytes. It is thought that the embryonic fibroblasts serve a large role in the formation of mature vasculature [5]. First, the fibroblasts lay extracellular matrix throughout the scaffold, the fibroblasts were found to differentiate into vascular smooth muscle cells which provides physical integrity to a mature vessel, and finally the triculture system had a high expression of three crucial angiogenic growth factors : VEGF, PDGF, and Angiopoitein-1 [5]. This study showed the importance of incorporating multiple cell types to create a functional tissue engineering construct [5].
Next, after implantation, it is crucial for the construct to anastomose with the host vasculature and form mature cardiac myocytes [5]. Additionally, the constructs cardiac myocytes need to synchronize with that of the host to avoid the construct from contracting independently from the rest of the heart. Lesman found that these prevascularized constructs were able to generate mature cardiac myocytes that contained gap junctions [5]. However, this degree of maturation varied greatly from rat to rat. Some possible thoughts were that the success of maturation depended on the degree at which the construct was vascularized [5]. Lesman also analyzed mature cardiac myocyte markers in the scaffold, and found that the triculture system had marked upregulation of these markers, which was not shown in the culture that only used cardiac myocytes as opposed to the triculture [5].
Cell sources:
As demonstrated by the previously displayed research there are a variety of cell sources that scientist have used in cardiac tissue engineering. Below are a list of the some of the most used cell sources [10]:
-
1.Fetal cardiomyocytes
-
2.Stem cell-derived embryonic cardiomyocytes
-
3.Skeletal myoblasts
-
4.Bone marrow mononuclear cells
-
5.Bone marrow-derived hematopoietic stem cells
-
6.Mesenchymal stem cells
-
7.Fibroblasts
-
8.Genetically engineering fibroblasts
-
9.Umbilical cord blood-derived cells
Biomaterials:
As demonstrated by the previously displayed research there are a variety of biomaterials that scientist have used in cardiac tissue engineering. Below are a list of the some of the most used biomaterial scaffolds [10]:
-
1.PLA
-
2.PLG
-
3.PLGA
-
4.Collagen
-
5.Fibronectin
-
6.Alginate

Figure 7: Dermagraft 2”x3” sample used for one time application.
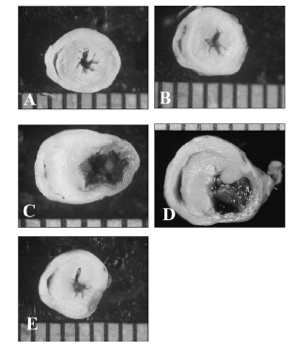
Figure 8: A) Normal mouse heart B) Normal rat heart with 3DFC treatment C) Infarct ONLY heart D) INFARCT heart with nonviable 3DFC treatment E) Infarcted heart with SDFC treatment.
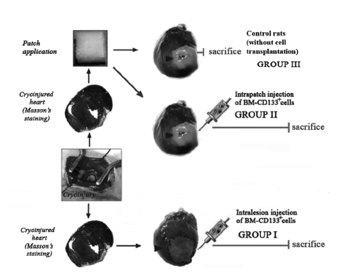
Figure 9: Schematic for scientific approach demonstrating the control, patch, and direct injection methods.
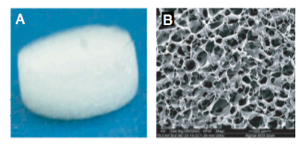
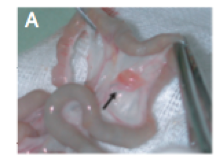
Figure 10: Images of the scaffold before cell implantation A) macroscopic view B) microscopic view of the porous nature of the scaffold
Figure 11: Image of the cardiac patch stitched within the rat omentum. Here we can see the host blood vessels entering the cardiac patch seven days after transplantation onto the omentum.
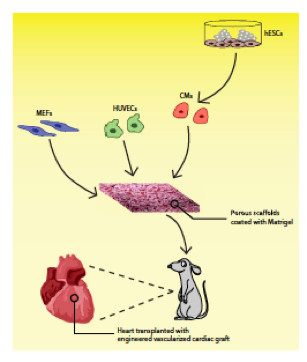
Figure 12: Schematic of the triculture approach in a porous scaffold coated with Matrigel as a cardiac patch.