Overall Design Criteria for ECoG Arrays:
The following design criteria is generally used in the design and manufacturing of an ECoG array to be implanted in the brain:
Materials Used: Stainless steel, carbon tip, or gold ball electrode
Number of Electrodes: 16 is the most common, 4 to 64 electrode contact range.
Sizes: Electrodes are 5 mm in diameter
Spacing: Standard spacing between grid electrodes is 1 cm
Recording Bandpass: 1-70 Hz, and avoiding 60 Hz notch filters
Important Frequency Bands: alpha- 8-12 Hz, beta- 18-26 Hz, gamma- >30 Hz



Figure 6: Different Types of Electrode Grids, Strips, and Tubular Electrodes for Epilepsy Surgery (10)
The above figure shows a typical electrode grid for ECoG to be used for epilepsy surgery. Note that these grids can be designed for each patient and each surgery type, depending on the location of the epileptogenic zone/lesion.
ECoG Seizure Detection Algorithms:
Besides requiring the specifications of ECoG arrays to be improved by improving the material, spacing, and overall size of each electrode, the seizure detection algorithms for surgeons to temporally determine epileptogenic zones for resection surgery are also important. Shown below in Figure 7, a typical seizure detection algorithm used for extraoperative surgery involves the complex techniques involved for an automatic seizure detection algorithm. Since it is impossible to know when a seizure will occur, normal seizure detection algorithms still involve human review of video and ECoG recordings to detect and score a seizure. Detection algorithms like the one shown below are an integral for ECoG recordings to be useful for defining epileptogenic zones.
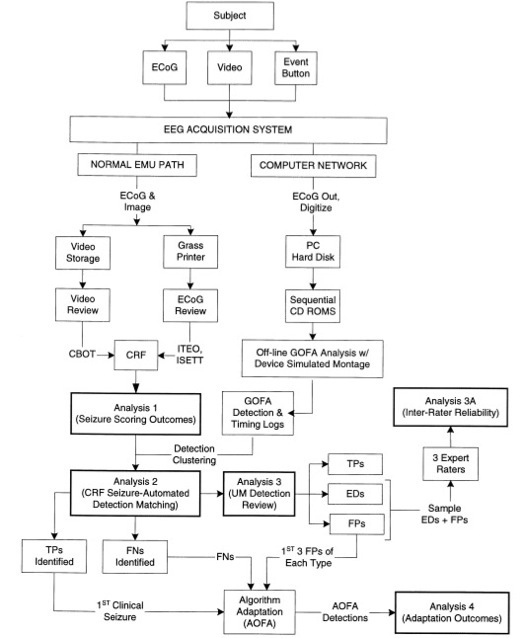
Figure 7: Real-Time Seizure Detection Algorithm (11)
ECoG Spike Detection and Seizure Detection:
There are still many issues with seizure detection algorithms for determining epileptogenic zones. First, there is an issue of too many false positives. Since other activities may cause recordings to have similar features to the recordings during a seizure, the algorithm may detect more seizures then actually occurred. This is an issue for surgeons as it does not automatically detect only seizures, allowing a localized spatial zone to be determined in a temporal setting. The following figures and video shows how seizures are detected and how ECoG recordings can detect differences between seizures and other responses of the brain.
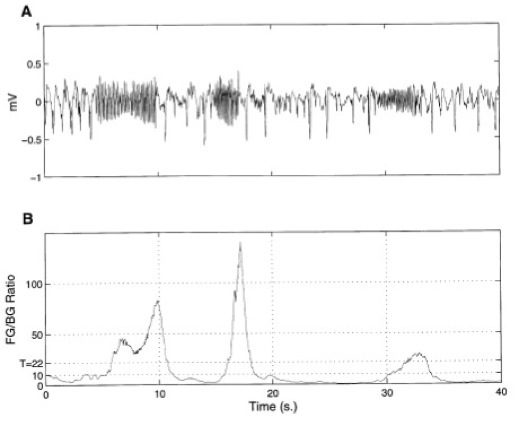
Figure 8: (A) Typical ECoG Channel Displaying a Discontinuous (on-off) Seizure and
(B) Generic Seizure Detection Algorithm Output (11)
It can be seen from the figure and video above that it is possible to detect seizures automatically as well as determine, to the millimeter, the location of seizure or brain activity. This allows for relatively accurate seizure detection and localization of epileptogenic zones. Thus, ECoG can be designed to fit each patient, allowing the epileptogenic zone to be determined accurately and the resection surgery to have little neurological damage from removing the lesion.
Limitations of Seizure Detection and Localization of Epileptogenic Zones Using ECoG:
Even though ECoG has proven to be the “gold standard” for defining epileptogenic zones and seizure detection over EEG, MRI and MEG techniques, there are still many limitations to using ECoG over these other techniques (especially new techniques such as SPECT and PET studies). Below are a few of the limitations in the design of ECoG, which engineers should try to overcome when designing an ECoG based system for detecting epileptogenic zones for resection surgery:
-
(1) The irritative zone is often more extensive the the epileptogenic zone, and the ECoG recordings cannot differentiate between the two zones (12)
-
(2) It is still difficult for ECoG to detect partial seizures
-
(3) The ECoG itself is invasive and is best used as an extraoperative surgery, which gives the limitation of two surgical procedures required
-
(4) Spatial sampling is highly limited, as ECoG currently only detects up to a millimeter and are usually spaced 1 cm apart.
-
(5)Anesthesia used during the surgeries can suppress spikes and induce burst-suppression, causing intraoperative mapping to be more difficult (12)
-
(6) There is limited duration of surgery, so if you’re looking for seizure activity during intraoperative surgery to map the epileptogenic zone using ECoG, you may not see any
-
(7) There is limited view of the brain during the surgery and limited locations for ECoG placement.
In order to overcome these limitations and several of the disadvantages previously explained in the Current Approaches section, engineers must develop better electrodes for neurological recordings and develop better seizure detection and epileptogenic zone localization techniques. ECoG and other imaging techniques can help perfect the resection surgery for intractable epilepsy, allowing better health for the over 50 million people worldwide that have epilepsy. The biomedical engineer must be aware of all current techniques and combine them accordingly to best help the surgeon resect the epileptogenic zone. Future work with ECoG, seizure detection, and defining epileptogenic zones that differentiate from irritative zones will also help surgeons resect epileptogenic lesions to improve the quality of life for patients with intractable epilepsy. Finally, biomedical engineers must further develop and design better signal processing systems to further assist neurologists in defining epileptogenic zones.