
Soft-Inertial Microfluidics
A shortfall of the MMBCD is that it requires the identification of the microbial prior to use. Microfluidics can also be used for separation and identification of microbials from blood samples based on soft-inertial methods. An example of this is the device designed by Klas Hjort’s lab out of Sweden. Exploiting the common size differential found between bacteria and red blood cells, soft-inertial methods refer to using momentum to separate the smaller bacteria from larger red blood cells.
INTRODUCTION
THEORY
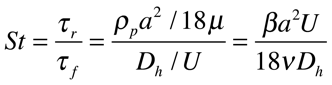
The basis of soft-inertia separation is based around the target particles (bacteria) Stokes number. The Stokes number, to the right, describes how quickly a particle adjusts to changes in the surrounding flow.
Where Beta is the ratio of fluid to particle density, a is the diameter of particle, U is avg. velocity in the channel, v is viscosity of fluid and D is diameter of channel.
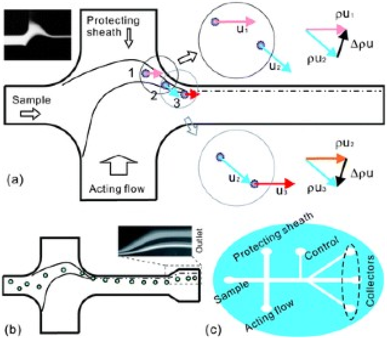
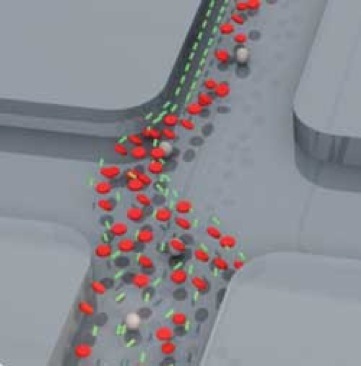
A larger St means that the particle has a stronger preference to continue with its original velocity direction rather than following the fluid trajectory. Keeping the stokes number in mind, researchers with Hjort’s lab were able to design a device that exploited the size differential between bacteria and red blood cells. In part (a) of the diagram to the right, the schematic of the device is shown. Following a sample fluid flow from the left, a separately controlled and much broader acting flow from below intersects with the sample flow perpendicularly. The result is a narrowing and acceleration of the sample flow that is outlined in (b). A third flow, labeled the protecting sheath, was added to prevent the cells from colliding with the curved radius of the device. A particle flowing within the sample flow path experiences a major momentum change at point 3 in (a). This momentum change, allows larger particles (red blood cells) to escape the fluid trajectory and form a collection stream further from the wall of the collection channel. Smaller particles (bacteria) continue to follow the fluid trajectory and can be collected close to the wall in outlets at the end of the device (c). A control flow was added to finely adjust the fluid trajectory for maximum collection.
CONCLUSION:
The device fabricated by Hjort’s lab was able to efficiently and cost effectively separate 62% of the bacteria from a small sample of blood. Such a device could lead to the high throughput collection of bacteria from a blood sample for diagnosis of Sepsis without requiring time intensive cell culture.