The Biofuel Cells
Biofuel Cells Employing Mediators to Facilitate the Biocatalyst-Electrode Electron Transfer
One approach to the design of an implantable biofuel cell prototype is to exploit the oxidation of a biofuel, such as glucose, coupled to the reduction of dissolved O2. This may be accomplished by mediated electron transfer from glucose oxidase at the anode [1, 12] to laccase [17,18] (or bilirubin oxidase [19]) at the cathode. The power output of the cell is the product of the cell voltage, Vcell and the cell current Icell. A redox mediator of appropriate redox potential is required to shuttle electrons between the protein and the electrode surface, because direct electron transfer to buried redox sites within these proteins is generally not possible given the distance of the active site from the electrode surface. This reduces the cell output voltage. However, for a glucose/O2 biofuel cell a Vcell higher than 0.5 V can be achieved by the utilization of enzymes and redox mediators of carefully chosen redox potentials. Biofuel cells operating with voltage outputs in the 0.5–0.8 V range have been reported previously [12]. The mediators and enzymes can be immobilized on electrodes by, among others, codeposition of redox polymer/enzyme and a bifunctional linker, co-electrodeposition and enzyme reconstitution at a functionalized electrode surface to provide membrane-less biofuel cells. The immobilization concept represents an important advancement, as it precludes the need to separate the anode and cathode half-cells from each other using a membrane, provided that no solution redox reaction between fuel and oxygen occurs (as is the case for glucose).
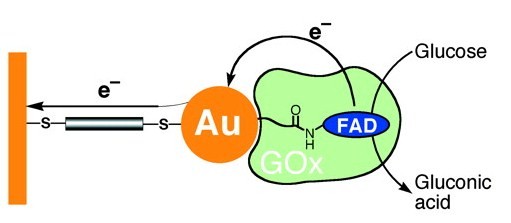
| Figure 2. Nanowiring of Redox Enzymes by a Gold Nanoparticle |
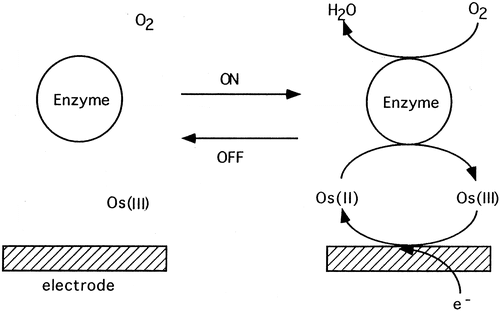
Figure 3. Catalytic scheme for the activity of the coimmobilized laccase and osmium redox mediator in a hydrogel on a glassy carbon electrode surface. |
Biofuel Cells Based on Direct Electron Transfer between the Biocatalysts and the Electrode Surfaces
between redox proteins and electrode surfaces. Research in interface design is slowly shifting from modifying the
electrode surfaces towards engineering of redox proteins. Protein engineering, which encompasses rational design, directed evolution and combined methods, offers many powerful methods and strategies for improving the electron transfer properties of redox proteins. Based on the theory of Marcus, three factors determine the rate of electron transfer from proteins, including the reorganization energies (qualitatively reflecting the structural rigidity in the oxidized and reduced form), the potential differences and orientations of the involved redox-active sites, and the distances between the redox-active sites and the intervening medium. The electrode and the protein active site can be regarded as electron donor–acceptor pair, where the protein or glycoprotein shell surrounding the active site becomes an effective kinetic barrier for the heterogeneous electron transfer, interfering with the efficient electrical communication with the electrode surface. Recently, direct electrochemistry of enzymes immobilized on electrode surfaces has attracted significant attention. Understanding the electron transfer reactions between an immobilized enzyme and an electrode surface can provide fundamental insight into physiological electron transfer processes as well as impetus for the further development of bioelectrocatalytic systems. Unfortunately, it is difficult for enzymes to exchange electrons with electrode surfaces directly, due to their large and complex structures, with their redox centers being deeply entrapped into their bodies ([12]. Consequently, covalently linked mediators were employed to improve the electron contact between enzymes and electrodes . Achieving such direct electron transfer has many practical applications, including the development of reagentless biosensors and biofuel cells, along with the increase of the rate of electron transfer between the enzyme and the electrode surface, increasing the yield of energy production of the respective biofuel cells.
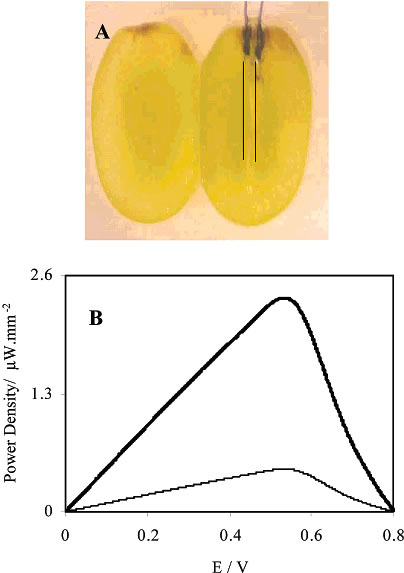
| Figure 4. (A) Photographs of a whole and a sliced grape with the implanted fibers and their electrical contacts. Because in the photographs the 7-ímdiameter fibers were barely visible, lines are drawn to show their positions. (B) Dependence of the power output on the cell voltage with the cathode fiber implanted near the skin of the grape (bold line) and near the center of the grape (fine line). |
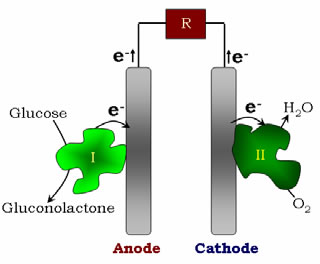
Figure 5. Biofuel cell configuration, with the electrons flowing from the anode to the cathode of the cell in a direct electron transfer system |