Background
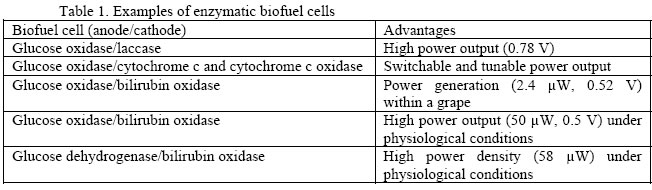
The potential mechanisms of biofuel cells involve the oxidation of a substrate by microorganisms or enzymes generating electrons in an external circuit. Since the first enzyme-based glucose/O2 biofuel cell operating at neutral pH has been studied by Yahiro et al. in 1964 [1], there have been two types of biofuel cells under ambitious research and development: microbial-based biofuel cells [2-5] and enzymatic based biofuel cells [6-9]. The biocatalyzed oxidation of organic substances by oxygen or other oxidizers in a two-electrode cell provides a potential means for the conversion of chemical to electrical energy in the body of a living subject. Abundant organic raw materials, like amino acids or glucose, can be used as substrates for the oxidation process, and molecular oxygen can act as the substrate being reduced. Like conventional fuel cells (such as: methanol fuel cells), which can deliver more energy per volume weight than conventional batteries, the theoretical efficiency of biofuel cells is also estimated to be as high as 90% [10]. If the biofuel cells use concentrated sources of chemical energy, they can be made safe, small and light. Moreover, biofuel cells differ from the traditional fuel cells by the material used to catalyze the electrochemical reaction. Instead of using precious metals as catalysts, biofuel cells rely on biological molecules, such as enzymes, to carry out the reaction. Currently the major thrust is in micro/nanostructured enzymatic biofuel cells and not in microbial-based biofuel cells because (1) current microbial based biofuel cells have several disadvantages: complex design, big size, poor reproducibility and reduced longevity of bacteria; (2) unlike conventional fuel cells that need periodic refueling, the implanted micro enzymatic biofuel cells could continue to produce electricity as long as the the biological host is alive; (3) instead of microorganisms, redox enzymes facilitate the electron transfer between substrates and electrode surfaces, thereby enhancing the cell current; (4) redox enzymes and bioelectrocatalysts can be easily immobilized on electrode surfaces; and (5) enzymatic biofuel cells can be operated under mild conditions with a simple electrochemical cell structure. Despite the many advances in enzyme- and polymer membrane modified electrode techniques, current enzymatic biofuel cells cannot compete with conventional batteries because of their low cell voltages and power densities. In addition, other key challenges of enzymatic biofuel cells include an uncertain storage and operational stability, tedious pretreatments of special and expensive electrode materials with a subsequent complex chemical and/or electrochemical functionalization, the adsorption of many interfering species on the electrodes, etc. Moreover, most of the redox enzymes lack direct electrical communication with electrode supports, and various electron mediators have to be used to contact the biocatalyst electrically with the electrode [11]. Some of the schemes for enzymatic microbial biofuel cell has been summarized below: