|
CLINICAL STUDY |
|
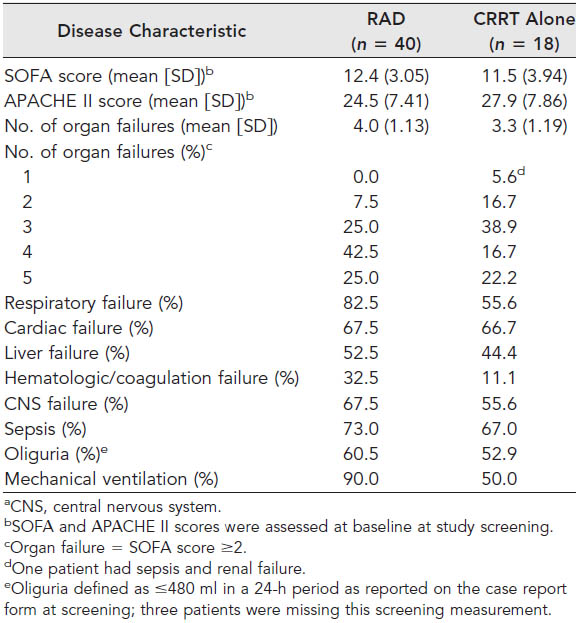
Tumlin et. Al details the results of the Phase II Clinical Study in “Efficacy and Safety of Renal Tubule Cell Therapy for Acute Renal Failure.” The RAD device was tested against standard continuous renal replacement therapy (CRRT). The RAD device was expected to provide additional components of loss kidney function that the CRRT could not such as its absorptive, metabolic, endocrine, and immunologic roles. The patients of both groups were in critical condition and suffered from a variety of serious health defects. RAD group was actually at a disadvantage as it's patients had a somewhat higher rate of organ failures than the CRRT alone group.
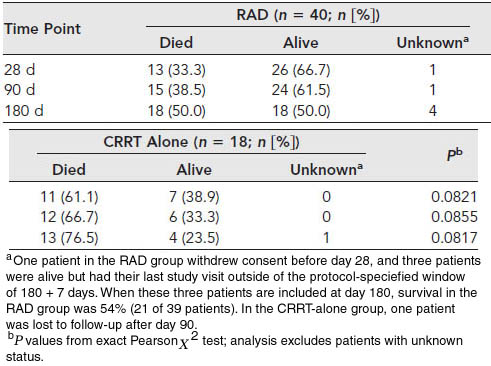
The table above shows the number of patients that lived or died after 28, 90, or 180 days due to all causes.
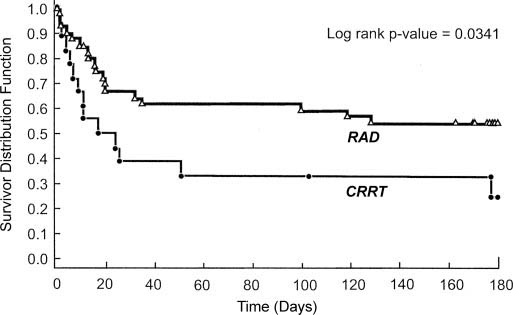
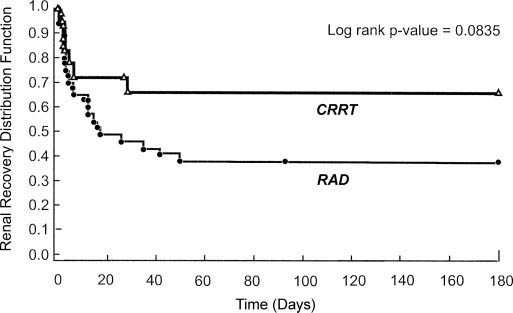
At the primary endpoint of 28 days, the mortality rate of the RAD group was 33%, whereas the rate of the control group was 61%. The RAD group was also more likely to survive through 180 days and saw more rapid recovery of kidney function as seen in the graphs of Kaplan-Meier estimates of survival below.
The graphs above display the survival function of the particular variable. The first graph is simply the number of patients surviving. The second graph measures the number of patients who have yet to recover renal function.
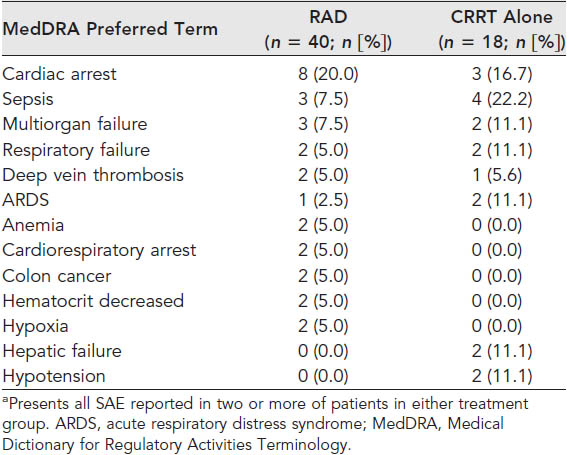
The severe adverse events (SAE) were reported in 68% of RAD patients and 89% of patients in the CRRT alone group. These numbers were within the expected range of extremely ill patients with ACF in an intensive care unit. Clearly the evidence supports the efficacy and safety of this treatment. The number of test subjects were low and only 12 medical centers were involved but this evidence could provide the needed data to validate the pursuit of a much larger Phase III trial which could lead to mainstream use. |
Website by David Dyer | UC Irvine BME240 | last updated 06/12/2009