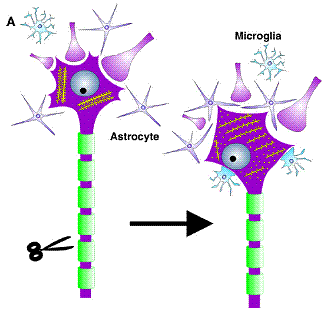
Mechanism of
regeneration

Schematic summary of cellular and ultrastructural changes following axonal injury.
Axotomy causes a detachment of neurite terminals and filling in of empty cell surfaces with CNS glia.
Inside the neuron, there is a
rearrangement of rough endoplasmitic reticulum cisternae or Nissl bodies
(Raivich et.al.).
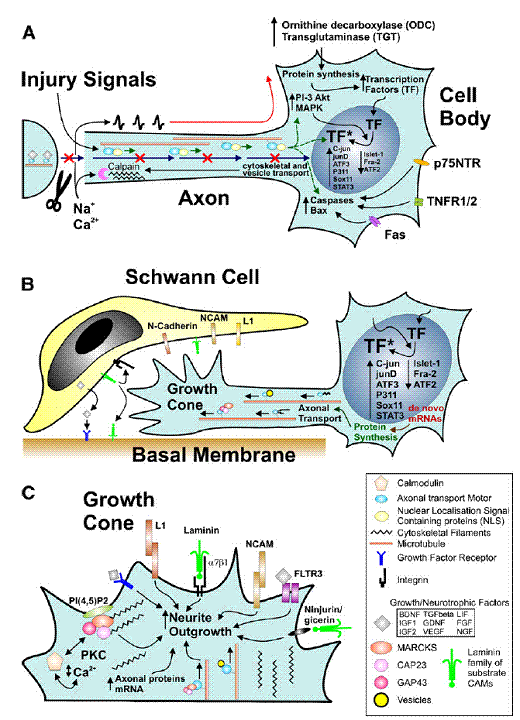
(Raivich G.
et.al)– Summary of cellular mechanisms involved in successful regeneration:
injury-induced signaling (A); cell body response involved in axonal growth (B);
and molecular mechanisms of neurite outgrowth at the growth cone (C).
(A)
Axonal
trauma induces a cessation of normal trophic retrograde transport (blue
arrows), a rapid influx of cations resulting in an antidromal train of action
potentials (red), and exposure to injury signals transported retrogradely via
proteins containing nuclear localization signals (green), that may act in synergy
to induce the biochemical activation in the neuronal cell body.
Ø
This
regeneration programme includes enzymes regulating mRNA metabolism—ODC, TGT,
intracellular cascades such as MAP kinases and PI3K-Akt, and complemented by
enhanced nuclear localization and phosphorylation or other forms of activation
(*)for a host of transcription factors (TF) including c-jun, etc., as well as
numerous molecules that regulate cell death, including death domain carrying
membrane proteins (p75, TNFR, fas) as well as cytoplasmic caspases and bcl-2
family members.
Ø
At
the cut axon, the rapid influx of Ca2+ causes calpain activation and
cytoskeletal remodeling that results in structured accumulation of
anterogradely transported vesicles and cytoskeletal components, transforming
the axon tip into an extension-competent growth cone.
(B) Regenerating neurons undergo
transcription factor-dependent changes in mRNA and protein synthesis that
result in the production and anterograde transport of vesicles and proteins
(cell adhesion molecules, cytoskeleton, growth-associated proteins such as the
GMC family members GAP43, CAP23 and MARCKS), that are needed for interacting
with the local cell adhesion and guidance molecules (L1, NCAM, N-cadherin,
laminin etc) and extending the axonal growth cone along the Schwann cells and
the basal membrane substrate of the peripheral neural tubes.
(C) A brief overview of signals regulating
neurite outgrowth at the axonal growth cone. This can involve a variety of
interactions via preferentially homophilic (L1, NCAM) or heterophilic cell
adhesion ligands (laminin, alpha7 beta1 integrin, ninjurin/gicerin), a variety
of growth factors and their receptors such as FGF and FLTR3, and the
phosphoinositol-4,5-diphosphates – PI(4,5)P2 – that associate with the GMC
family of calcium/calmodulin ligands and Protein Kinase C (PKC) and regulate
actin cytoskeleton polymerization, organization and disassembly. The
anterograde axonal transport of vesicles, signaling molecules, cytoskeletal
proteins and their mRNA plays a critical role in providing structural and
regulatory components for axonal elongation.