Synthetic ACL Replacements
Website made by Shia-Yen Teh ( 2006)
Purely Synthetic Grafts
Synthetic ACL prostheses were first considered due to the ease of manipulating their mechanical properties. The prostheses fabricated had mechanical properties equaling or exceeding those of a normal ACL. In general, due to the stiffness of the material, synthetic grafts provided sufficient initial strength and had excellent short-term results, but in the long-term, many patients encountered fatigue failures and poor integration of the graft with the host.
One of the first examples of a synthetic replacement that was FDA approved, was the Gore-Tex ligament, the trade name for expanded polytetrafluoroethylene (PTFE). Introduced in 1982, it was used in humans for about 10 years, but was discontinued due to pain, effusion, and instability experienced by the patient postoperatively. The graft had an ultimate strength of 4800N, and other similar mechanical properties as an ACL. The graft is made of braided PTFE, which is an inert polyethylene and has a sheathed multifilament structure which aids in increasing its tensile strength.
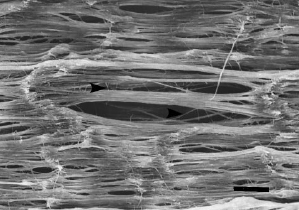
SEM image of a Gore-Tex ligament. Arrow points to a 0.1μm diameter fiber. (IW Forster, 1998)However, a study done by Ian Forster and his group at the Queen’s Medical Centre in the UK, showed that Gore-Tex caused strong immunological reactions. From wear and tear, the graft formed small particles which brought about biological activity, inflammation, and attack by macrophages. The study also found that the Gore-Tex particles accumulated in both local and distal lymph nodes. The graft was discontinued in 1993 due to its poor performance, high rupture rates, painful side effects, and cost.
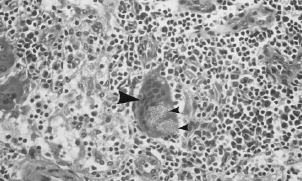
Lymph node section showing Gore-Tex particles (small arrow) in a cell (large arrow), (IW Forster, 1998)Other synthetic grafts such as carbon-based prostheses, the Leeds-Keio (polyethylene terephthalate), and the Stryker-Dacron ligament have had similar fates due to the wearing off of particles into the knee, inflammatory responses, cartilage degeneration, and poor functional stability. The Stryker-Dacron replacement was never FDA approved, most likely due to its poor clinical results. In a 4 year follow up study, it was found that 28% of the Dacron prostheses ruptured, and that degenerative arthritis was found in all the patients with the ruptured replacement.
Tissue In-growth in Non-degradable Scaffolds
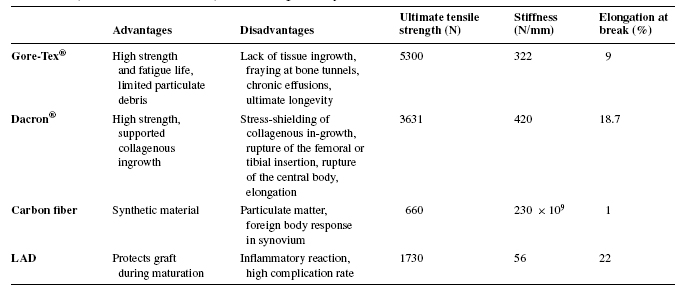
More advanced synthetic prostheses constructed of polymers or carbon fibers were designed to promote tissue growth within the graft. The grafts were made into porous or filamentous structures so as to allow for room for cell proliferation. One of the first companies to develop this graft was 3M, which created the Kennedy Ligament Augmentation Device (LAD). This material was a hybrid of the polymer, polypropylene, and host tissue. However because the graft was non-degradable and had relatively high rupture rates, like the previous synthetic materials, this hybrid graft also developed inflammatory responses and painful side effects. Many of these tissue in-growth designs have failed because the tissue grows in a disorganized fashion and often resembles scar tissue. This is due to the fact that the stiffness in the polymer is much higher than that of the growing tissue, thus much of the loads are placed on the graft, causing stress-shielding effects to prevent proper remodeling of the tissue. The ideal tissue in-growth graft should also be able to remodel in response to long-term effects of the imposed loads. The table below shows measured ultimate strength and stiffness of various synthetic grafts (Note the differences between those values and that of natural ACL which has ultimate strength of 2000N and stiffness of 240N/mm)
Mechanical properties of synthetic ACL replacements (PP Weitzel et al, 2002)
Tissue In-growth in Biodegradable Scaffolds
Current synthetic graft research is focused on biodegradable polymers. These scaffolds are more biocompatible than other synthetic materials because the polymers can be slowly resorbed by the body and replaced with tissue in-growth. A number of different biodegradeable polymers exist; each polymer having different rates of degradation, mechanical properties, and cellular response. Laurecin et al. created a braided poly(lactic-co-glycolic acid) (PLGA) scaffold with various sized pores for bone and ligament tissue growth. In vivo tests showed that the scaffolds were capable of supporting both rabbit and murine ACL fibroblasts. The addition of cell adhesion molecules such as fibronectin may enhance cell binding and proliferation to the polymer.
A 15 year study done by BC Frank et al, found that of 855 synthetic replacements, 40-78% failed due to the accumulation of debris, mechanical failure, or immunological reactions. In general, the rates of synovitis, arthritis, and wearing of the ACL prostheses worsen with time. Thus, alternative ACL replacement materials are needed that can parallel the strength of native ACL, yet maintain long term functional capabilities.