|
RENAL TUBULE CELL ASSIST DEVICE (RAD) |
|
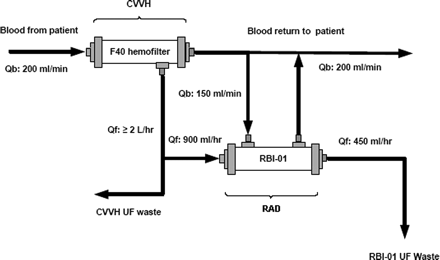
Proximal tube cells are harvested from cadaveric renal donors ineligible for kidney transplantation. About 0.5 to 1.0 x 108 cells are grown on the insides of hollow fibers that form a standard hemofiltration cartridge. This fibers not only serve as a substrate for the cells but as protection. Since the cells are nonautologous, they must be protected from the patient's immune system during the filtration process. The pore size created by the weave of the fibers and the fact that it is nonbiodegradable ensures that the cells are preserved. This extracorporeal device has been dubbed the renal tubule cell assist device (RAD) by its developers in Dr. Humes's laboratory at the University of Michigan. The cells in this device had been shown to keep transport, metabolic, and endocrine activities in preclinical studies. When placed in series with another hemofiltration device in treating animals with damaged kidneys, those functions were retained as well as the filtration expected from dialysis. It was shown to lessen multiorgan dysfunction in Gram-negative septic shock in large-animal models. The treatment also progressed to open-label Phase I/II human clinical trails where it not only proved to be safe to administer in conjunction with continuous venovenous hemofiltration for 24 hours but dramatically improved some of the organ systems of the patients and decreased their 30-day mortality. An even later Phase II clinical trial demonstrated safe use for up 72 hours and showed further improved survival rates allowing for the possibility of a Phase III randomized and multicenter trial.
|
Website by David Dyer | UC Irvine BME240 | last updated 06/12/2009