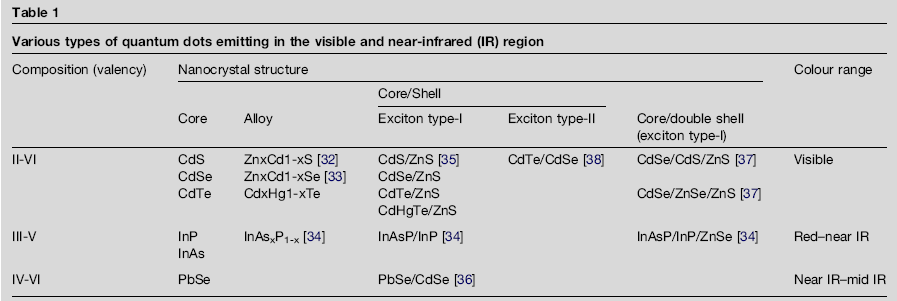
| Quantum dots are labeled by composition, core/shell structure, and type of excitation [7]. Compounds and alloys of II-VI emit in the visible light range, III-V emit in the red to near infrared, and lastly IV-VI in the infrared (Fig. 1) [7]. The core/ shell structure increases the quantum yield and the stability of the dots [7]. A type-I structure contains a core where both the electrons and holes are confined to the core and the shell acts to protect the core from the medium [8]. A type-II structure consists of a core where the conduction and valence bands are lower or higher than the shell which results in one charge carrier in the core and the other confined to the shell [8].
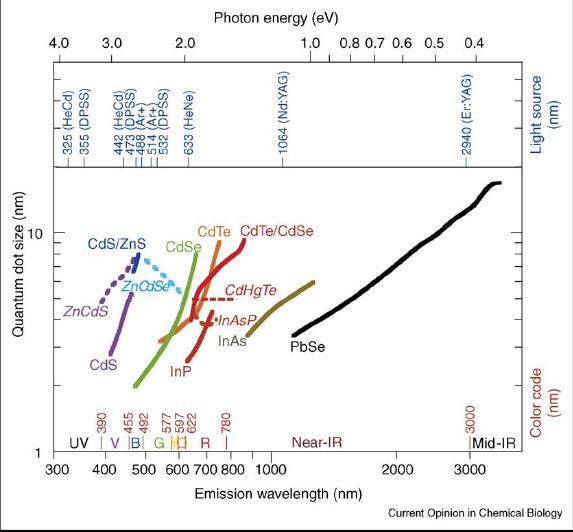
Fig, 1 Table of various quantum dots. Depending on the composition and size of the quantum dots, different optical properties can be tuned (Fig. 2).
Fig. 2 Variable fluorescence of quantum dots due to their size and composition. 1. Organic synthesis Bawandi et al first developed this method using dimethyl cadmium Cd(CH3)2 which acts as the cadmium precursor [9]. The method entails pyrolysis to make quantum dots by injecting organometallic reagents into hot coordination fluid (300˚C) such as triocytlphosphine (TOP) and triocytlphosphine oxide (TOPO), which are used as capping reagents and the reaction medium [2,9]. These hydrophobic organic molecules prevents the formation of bulk semiconductor formation by coordination with the unsaturated metal atoms on the surface of quantum dots [2]. The TOPO and TOP organic ligands in the inner surface of quantum dots are important for maintaining the optical properties of the dot and also protects the core from the medium [6,9]. The quantum yield of quantum dots made from this method range from 50% to 80% and the quantum dots have good crystallinity and are free of surface defects [9]. However Cd(CH3)2 is toxic, unstable, expensive, pyrophoric, and explosive at temperatures higher than room temperature which makes the fabrication of quantum dots dangerous and expensive [9]. Peng et al were able to improve upon the method of Bawandi by replacing Cd(CH3)2 with CdO as the Cd precursor which produced reproducible results favorable for industrial production scale up [9]. Using this method, addition of tellurium, selenium, and sulfur stock can yield CdTe, CdSe, and CdS quantum dots[9]. The organic synthesis of quantum dots also pose the problem of being insoluble in water and unable to be used in vivo [9]. The surface capping molecules such as TOPO needs to be replaced before their use in a biological system [9]. The size of the quantum dot is determined by the precursor amount and crystal growth time [2]. Watch quantum dots being made: http://mrsec.wisc.edu/Edetc/nanolab/CdSe/index.html 2. Traditional aqueous synthesis Aqueous synthesis entails mixing metallic salts such as cadmium salts with NaHTe or NaHSe and thiol compounds as capping reagents [9]. Quantum dots that are able to be fabricated using this method is CdSe, CdTe, and HgTe, but quantum yields for these dots are smaller compared to those prepared through organic synthesis [9]. Also the particles do not possess the high crystallinity seen in organically prepared quantum dots, but the procedure is simpler, inexpensive, and more reproducible than organic synthesis [9]. In addition to the above limitations of this technique, the method takes a long time in the reaction phase from about several hours to several days [9]. The synthesis time can be greatly shortened and the quantum yield increased by using higher temperatures utilizing such devices such as autoclaves and microwaves [9].
|