Damage control neurosurgery (DCNS) was developed to prevent secondary injuries from swelling and low blood
flow in patients with traumatic brain injury (TBI). According to Rocco Armonda at the National Naval Medical
Center, “If you can open the skull early enough to prevent secondary injuries like low blood flow, the chances
of recovery are better than ever.” Survival rates with this technique have increased from 10% to about 50%,
and also increased the number of patients who are able to function independently afterward.
DCNS works by removing a section of the patient’s skull to allow the brain to swell rather than putting pressure
on the brainstem and also evacuate any intracranial hematomas. Rather than relying on a single surgery, DCNS
follows up with micro-balloons and medications to ensure that blood flow is reaching the brain, and once
swelling abates, the bone flap or a hard acrylic implant is inserted in a cranioplasty procedure.
For this to be effective, time is of the essence, and the initial surgery should be done no more than 60 minutes
after the patient arrives. This rapid approach is what sets DCNS apart from standard neurosurgery procedures.
Fortunately, it is also a procedure that may be done in rural or military settings by a generalist when the
patient is far from a trauma center, with following care provided by a trauma team.
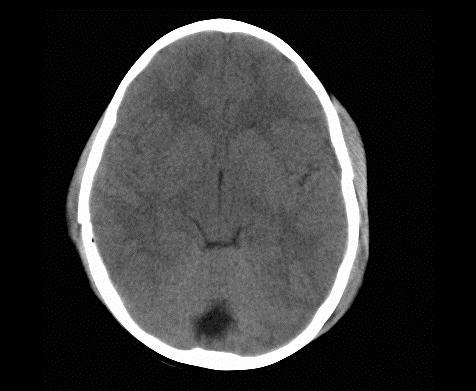
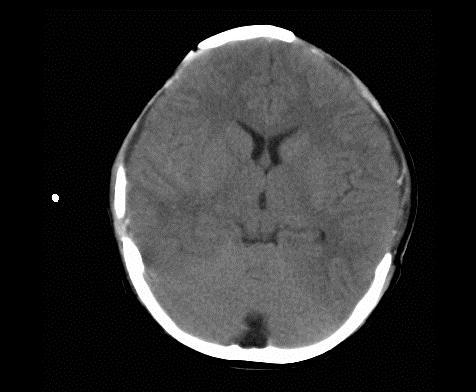
Below are images showing CTscans before and after a bilateral craniectomy. As the brain was able to expand
afterward, the intercranial pressure has been reduced. Images from Ruf, 2003.


In the early 1900’s, the concept of DCS had been created by Schroeder and Halstead, but the technology at the
time was not up to the task. It was attempted again during World War II, and finally in 1978, a temporary
packing method to prevent hepatic hemorrhage was found to be effective.
The general principles of damage control surgery (DCS) were published in 1993, consisting of laparotomy,
secondary resuscitation, and delayed definitive surgery (usually 24-72 hours later). In 2003, a team of eight
neurosurgeons and neurologists stationed near Fallujah decided to try this for traumatic brain injuries and
developed the DCNS procedure.
After being pioneered in the front lines of Iraq, this approach to caring for traumatic brain injuries is starting to
make inroads in the United States. This new technique is important, as 1.4 million Americans suffer such brain
injuries each year. Here, surgeons practice craniotomies on a cadaver (image from Rosenfeld, 2004).
There is a five-stage approach for DCS: patient selection, DCS, restoration of the physiology in the ICU,
definitive surgery, and cranioplasty.
In some cases, dural expansion may also be needed, and pericranium, temporalis fascia or synthetic dura can
be used to do this. Once the initial surgery is complete, care strategy is very similar to that given to stroke
patients, with micro-balloons and medications to increase blood flow, and approximately six weeks thereafter,
a plate or bone flap is inserted and the wound closed.
Craniectomy vs. craniotomy?These procedures are similar, but craniectomies remove part of the skull and craniotomies only use small holes or immediately replace the bone flap. |
Coning or dilated pupils and a low GCS score may indicate that a craniotomy should be performed immediately,
even if other procedures are being done simultaneously.
Intracranial hematomas would be located with a head CT scan so that craniotomies can be done in the correct
locations.
An intraparenchymal ICP monitor may be inserted by a neurosurgeon in the emergency room and used to
monitor ICP in the surgery and ICU as well (or inserted by a general surgeon in a remote military hospital).
Other means of reducing ICP include administering Mannitol and draining CSF fluid.
Focal brain oxygen tension is more accurate than mixed jugular valve oxygen saturation measurements. This
is monitored with probes within the cranium, so it is not used preoperatively, but can provide information on
oxygen saturation during care in the ICU.
In the future, a test for inflammatory mediators may provide additional information about whether the brain is
likely to have
swelling. The potential for therapeutic hypothermia in TBI
patients is also being evaluated.
http://www.usuhs.mil/alumni/innovations.pdf
I.K. Haitsma and A.I.R. Maas, Advanced monitoring in the intensive care unit: brain tissue oxygen tension. Curr. Opin. Crit. Care 8 (2002), pp. 115–120.
Hemphill, J.
Claude, III, Smith, Wade S., Sonne, D.
Christian, Morabito, Diane, Manley, Geoffrey T. Relationship
between Brain Tissue Oxygen Tension and CT Perfusion:
Feasibility and Initial Results Am J Neuroradiol
2005 26:
1095-1100.
Marius Keel, Ludwig Labler and Otmar Trentz. “Damage Control” in Severely Injured Patients Why, When, and How? European Journal of Trauma 31:3, June 2005
Maeyane S.
Moeng, Jerome A.
Loveland, and Kenneth D.
Boffard. Damage
Control:
Beyond the Limits of the Abdominal Cavity. A Review International TraumaCare
Fall 2005, pp. 189-196.
Jeffrey Rosenfeld. Damage control neurosurgery. Injury Volume 35, Issue 7, July 2004, Pages 655-660
Early
decompressive craniectomy and duraplasty for refractory intracranial
hypertension in children: results of a pilot study. Critical
Care
2003, 7:R133-R138