Artificial Oxygen
Carriers |
 |
The early development of the RBC substitutes in early 1900’s was initiated by the need of the military for an improved resuscitation fluid. |
> Not transmit disease
> Not have immunosuppressive effects
> Be available in abundant supply
> Be universally compatible
> Have prolonged shelf life
> Be stable at a range of temperatures
> Be available at a reasonable cost
> Be easy to administer
> Be able to access all areas of the human body
> Be effective at room air or ambient conditions
 |
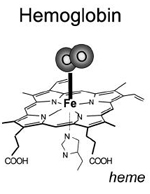 |
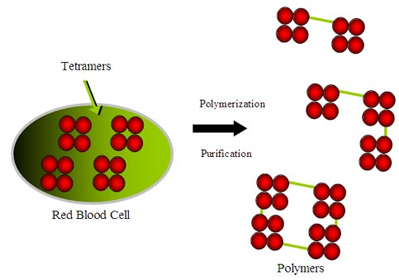
Hemoglobin-based oxygen carriers contain pure hemoglobins separated from the human and bovine RBCs. This acellular solution eliminates immunologic reactions by isolating hemoglobins from RBCs which contain antigens that determine bloodtype. However, the raw hemoglobin with α2β2 tetramer could not be used because it was dissociating into αβ dimers when tested, which could be filtered through renal glomeruli and cause renal toxicity. The challenge in creating a hemoglobin-based artificial blood is to modify the hemoglobin molecule so these problems are |
 |
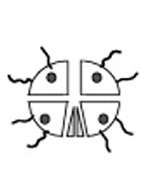 |
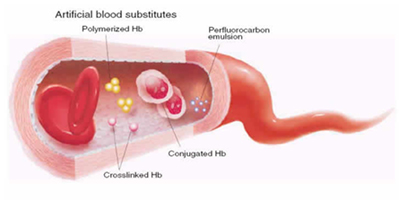
Conjugated Hb Conjugation of hemoglobin is the covalent binding of Hb to a biocompatible polymer, such as polysaccharide, in order to increase its overall size. Such a process achieves similar improvements as those made using polymerization. |
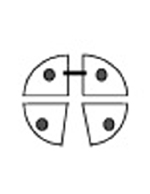 |
Crosslinked Hb Because the alpha/beta (α-β) dimers are relatively stable, the goal of intramolecular modification is to cross-link the two alpha (α-α) or beta (β-β) subunits and stabilize the association of the two alpha/beta (α-β) dimers. The cross-linking not only prevents tetramer dissociation, but also reduces the affinity of Hb for O2. Reagents such as o-raffinose or glutaraldehyde are crosslinked between amino groups (NH2) located on the surface of hemoglobin to form a stabilized tetramer and to prevent their dissociation into |
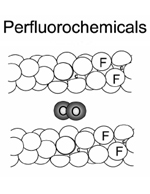 |
Perfluorocarbons are compounds that are biologically inert that can dissolve about 50 times more oxygen than blood plasma. Its chemical properties showing high O¡þ2 and CO2 dissolving capacity and low lipid and water solubilities make them ideal temporary intravenous oxygen carriers. PFCs exchange gases by simple diffusion and bind them by loose, non-directional van der Waals interactions. With PFCs, there is no saturation and no possibility for chemical binding of, and interference with, NO, CO or other reagents. PFC-dissolved O2 is immediately |