E-mail:
Currently, numerous research groups are focusing greatly on the right biomaterial to use as the scaffold for a nerve guidance channel. However, many incorporate other important mechanical and physiological properties, including degradation and drug delivery. Described below are some current advancements in nerve guidance channels, including:
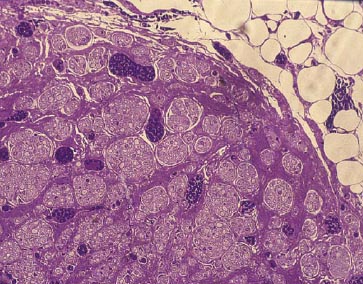
Lee et al. have developed a novel biomaterial scaffold that has been shown to have superior effect than vein grafts in rabbits. The novel biomaterial consists of a poly(L-lactide-co-glycolic acid)-coated collagen tube (Figure 1). The inside of the tube is filled with a collagen gel. To test their scaffold, a 12 week experiment showed morphologically and electrophysiologically that the novel biomaterial scaffold was similar, if not better, than the vein graft over a 15mm gap. As seen in Figure 2, there is no significant difference histologically between the nerve guidance channel and vein graft.8
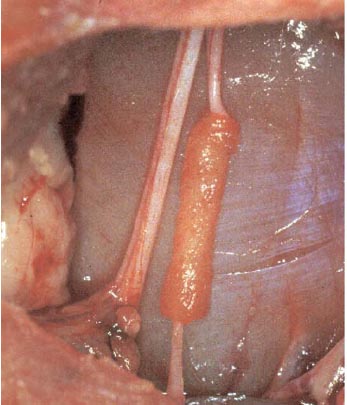
Figure 1. Poly(L-lactide-co-glycolic acid)-coated collagen tube.8
 |
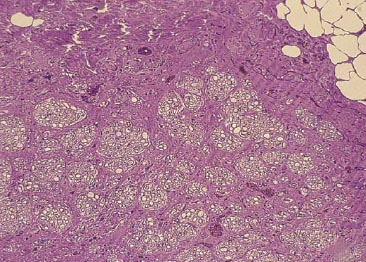 |
Figure 2a. Nerve guidance channel.8 Figure 2b. Vein Graft.8
Katayma et al. also created a novel biomaterial scaffold designed to be as good if not better than an autologous nerve graft. The group focused on optimizing the patency, or openness, of nerve guidance channels. As a result, their experiment compared the effects of a plain, corrugated, and coil-reinforced tube, as shown in Figure 3. From their experiments, the coil-reinforced tube proved best based on patency. In addition, they showed the coil-reinforced design proved superior electrophysiologically by having similar nerve action potential (NAP) and muscle action potential (MAP) velocity to autologous nerve grafts as well as by histomorphometry, by possessing similar axon density.9
 |
 |
Figure 3a. Corrugated design.9 Figure 3b. Coil reinforced design.9
Rutkowski et al have developed a poly(D-L-lactic acid) (PDLLA) with a micropatterned inner lumen, where Schwann cells can line the interior and offer more support for axonal regeneration, as depicted in Figure 4. In order to test their device, they compared the results of nerve regeneration in a rat with a 1cm gap to a guidance channel with micropatterning but no Schwann cells and a guidance channel with no micropatterning and no Schwann cells. Figure 5 shows the migration of the preseeded Schwann cells moving closer to the center of the lumen. The results showed that there is no significant change in regenerated axons; however, there is significantly better increase in recovery time and sciatic function index, which corresponds to a tracking system measuring the rat's walking.10
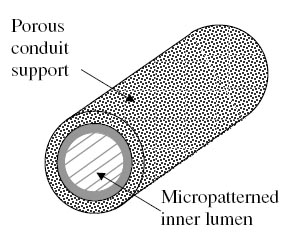
Figure 4. Porous guidance channel with interior micropatterned lumen.10
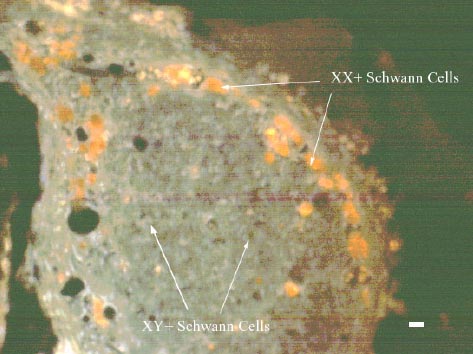
Figure 5. Preseeded Schwann cells are labeled XX+ (orange), while migrating Schwann cells are labeled XY+ (green-orange).10
Yang et al have developed a porous biomaterial scaffold capable of controlled release of neurotrophic factors, specifically NGF. As seen in Figure 6, this design incorporates multiple lumens and is created with poly(lactide-co-glycolide) (PLG) microspheres with porogen (NaCl). Numerous tests were conducted to find the best ratio between both materials to maintain porosity and mechanical properties. The growth factor, NGF, was either mixed with the microspheres or encapsulated within the microspheres. Experimental results showed that sustained release occurred for at least 42 days and NGF maintained is bioactivity, as indicated by stimulation of dorsal root ganglion. Experimental results in vivo showed that the nerve guidance channel maintained is mechanical properties and allowed for cellular migration into the inner lumen.11
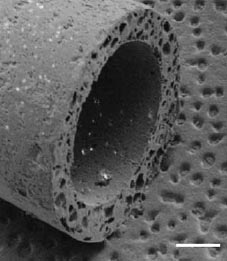 |
 |
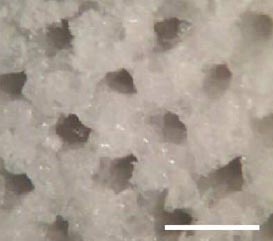 |
Figure 6a. SEM image.11 Figure 6b. Scaffold.11 Figure 6c. Magnified view.11