Typically, a sample is illuminated
with a laser beam. Light from the illuminated spot is
collected with a
lens and sent
through a monochromator. Wavelengths close to the laser
line (due to elastic
Rayleigh scattering)
are filtered out and those in a certain spectral window
away from the laser line are dispersed onto a detector.
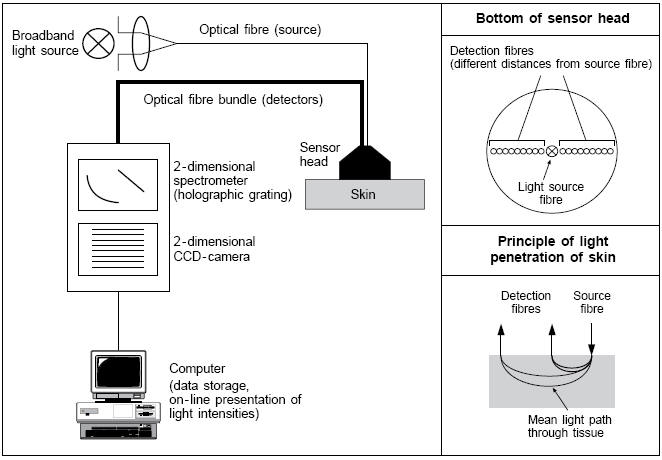
Spontaneous Raman scattering is typically very weak, and as a result the main difficulty of Raman spectroscopy is separating the weak non-elastically scattered light from the intense Rayleigh scattered laser light. Raman spectrometers typically use holographic diffraction gratings and multiple dispersion stages to achieve a high degree of laser rejection. A photon-counting photomultiplier tube (PMT) or, more commonly, a CCD camera is used to detect the Raman scattered light.3
The conventional Raman techniques for detection of
body level constituents are generally associated with
long exposure time (several ten minutes), high power
laser pump fluency which is much above the safety
limitation of the laser illumination for human body
applications, and strong noise background. Conventional
Raman is too weak to determine analyte (glucose) level
in humans due to the background.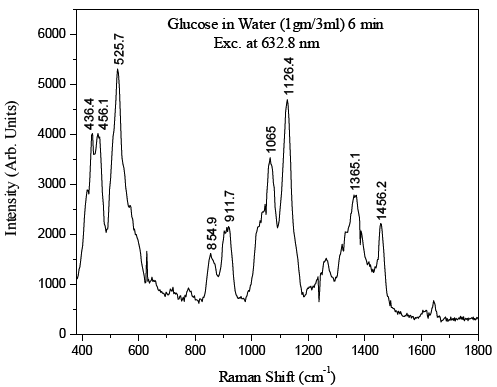
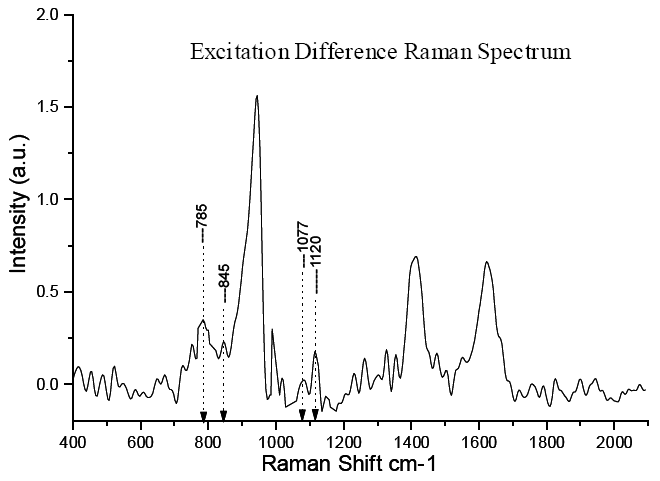
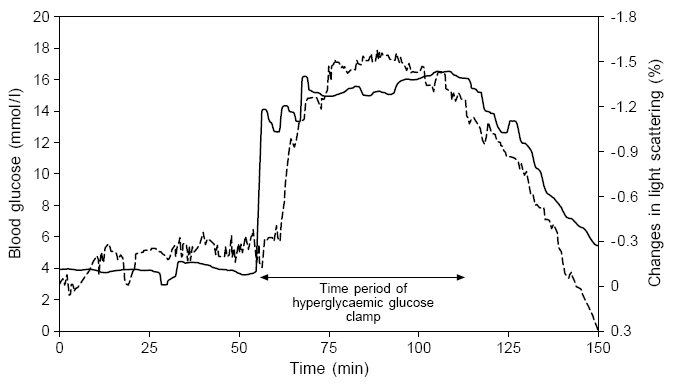
In order to apply Raman spectroscopy to achieve high sensitivity for glucose level detection for diabetes diagnosis, and other body level analyte detection in blood, we have investigated fingerprint Raman modes for glucose and other Raman-active blood analytes using a novel approach. A method called low power cw excitation raman spectroscopy as well as difference Raman spectroscopy were developed to find the body level glucose and enable a glucose fingerprint to be formed. Currently the figure to the right shows various viable "fingerprints" that can used. The figure below was obtained via a human fingertip. Thus showing the possibility of obtaining non-invasive blood glucose optically.3
¡@



 1
1