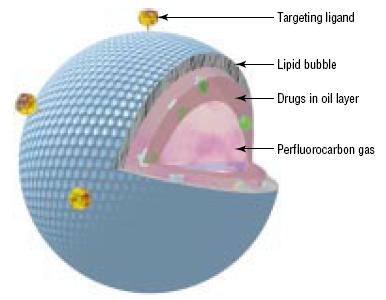
Targeted microbubbles have ligands that bind specific receptors expressed by cell types of interest, such
as inflamed cells or cancer cells. They are under preclinical development. Current microbubbles in
development are composed of a lipid monolayer shell with a perflurocarbon gas core. The lipid shell is
also covered with a polyethylene glycol (PEG) layer. PEG prevents microbubble aggregation and makes
the microbubble more non-reactive. It temporarily “hides” the microbubble from the immune system
uptake, increasing the amount of circulation time, and hence, imaging time. In addition to the PEG layer,
the shell is modified with molecules that allow for the attachment of ligands that bind certain receptors.
These ligands are attached to the microbubbles using carbodiimide, maleimide, or biotin-streptavidin
coupling. Biotin-streptavidin is the most popular coupling strategy because biotin’s affinity for
streptavidin is very strong and it is easy to label the ligands with biotin. Currently, these ligands are
monoclonal antibodies produced from animal cell cultures that bind specifically to receptors and
molecules expressed by the target cell type. Since the antibodies are not humanized, they will elicit an
immune response when used in human therapy. Humanizing antibodies is an expensive and time-intensive
process, so it would be ideal to find an alternative source of ligands, such as synthetically manufactured
targeting peptides that perform the same function, but without the immune issues.