[CURRENT RESEARCH]
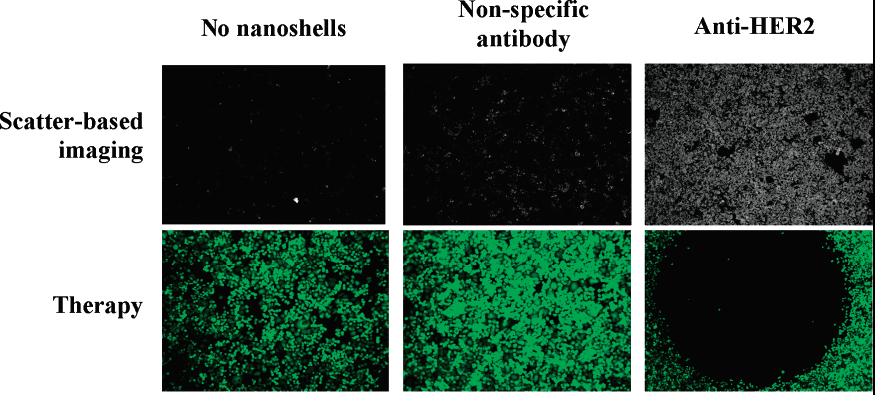
The efficacy of nanoshells as sensitizers has been analyzed in a couple of studies. First, an in vitro study demonstrated the ability of nanoshells to act as immunotargeted sensitizers. In a study by Loo et al., immunotargeted nanoshells were used to image and destroy breast carcinoma cells that over express HER2. The study was carried out using cell cultured tissue. Nanoshells were tuned to have the same scattering and absorption peaks in the near-infrared region. The images below demonstrate the impact of nanoshells in laser therapy. The images are taken via dark-field microscopy (a scattering based imaging modality). The top row represents cell before treatment and the bottom row post-treatment. More importantly, the two pictures in the right most column show the dramatic improvement possible by using nanoshells with specific antibody binding.[3] The top row demonstrates the improved accumulation of immunotargeted nanoshells. The bottom row provides visual evidence of improved selective photothermolysis of tumor cells based on the use of these immunotargeted nanoshells.
The impact of Immunotargeted Nanoshells

An in vivo study of mice tumors has also been carried out. In this study, PEG-coated nanoshells tuned to the near-infrared region were injected intravenously into mice with subcutaneous tumors of 3-5.5mm. After, the nanoshells were given sufficient time to accumulate in the tumor via the concept of enhanced permeability of retention (EPR), the mice were treated with a near-infrared laser and surface temperatures were monitored as well as chronic tumor growth. The study showed that mice that were treated with laser tumor ablation remained healthy and free of tumors. In contrast, the control group that received no treatment suffered from additional tumor growth. However, the study showed that EPR was not sufficient enough to ensure selectivity as tissue outside of the tumor was shown to have a thermal response. Thus, EPR is sufficient to generate a therapeutic response at the tumor site but not sufficient to affect damage to surrounding tissue.[4] The next step in research would be to combine the above two concepts. Specifically, the efficiency of immunotargeted nanoshells for selective photothermolysis should be studied in an in vivo model.